MonascinCAS# 21516-68-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

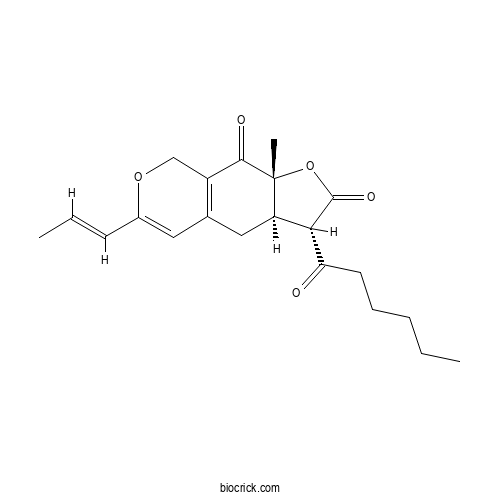
| Cas No. | 21516-68-7 | SDF | Download SDF |
| PubChem ID | 12118082.0 | Appearance | Powder |
| Formula | C21H26O5 | M.Wt | 358.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,3aR,9aR)-3-hexanoyl-9a-methyl-6-[(E)-prop-1-enyl]-3,3a,4,8-tetrahydrofuro[3,2-g]isochromene-2,9-dione | ||
| SMILES | CCCCCC(=O)C1C2CC3=C(COC(=C3)C=CC)C(=O)C2(OC1=O)C | ||
| Standard InChIKey | XXKNHBAFFJINCK-RVEJDSBJSA-N | ||
| Standard InChI | InChI=1S/C21H26O5/c1-4-6-7-9-17(22)18-16-11-13-10-14(8-5-2)25-12-15(13)19(23)21(16,3)26-20(18)24/h5,8,10,16,18H,4,6-7,9,11-12H2,1-3H3/b8-5+/t16-,18+,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Monascin Dilution Calculator

Monascin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7899 mL | 13.9497 mL | 27.8995 mL | 55.7989 mL | 69.7486 mL |
| 5 mM | 0.558 mL | 2.7899 mL | 5.5799 mL | 11.1598 mL | 13.9497 mL |
| 10 mM | 0.279 mL | 1.395 mL | 2.7899 mL | 5.5799 mL | 6.9749 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.558 mL | 1.116 mL | 1.395 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.558 mL | 0.6975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Thymoquinone
Catalog No.:BCX0807
CAS No.:490-91-5
- Neoeuonymine
Catalog No.:BCX0806
CAS No.:33510-25-7
- Cortodoxone
Catalog No.:BCX0805
CAS No.:152-58-9
- Cortisone
Catalog No.:BCX0804
CAS No.:53-06-5
- Quercetin 7-O-glucuronide
Catalog No.:BCX0803
CAS No.:38934-20-2
- Coreopsin
Catalog No.:BCX0802
CAS No.:499-29-6
- Harmalan
Catalog No.:BCX0801
CAS No.:525-41-7
- Prosaikogenin G
Catalog No.:BCX0800
CAS No.:99365-23-8
- Pterin-6-carboxylic acid
Catalog No.:BCX0799
CAS No.:948-60-7
- 17-Hydroxygracillin
Catalog No.:BCX0798
CAS No.:90308-85-3
- Kanzonol D
Catalog No.:BCX0797
CAS No.:155233-20-8
- (-)-Epipodophyllotoxin
Catalog No.:BCX0796
CAS No.:4375-07-9
- guan-fu base I
Catalog No.:BCX0809
CAS No.:110225-59-7
- Acetyl Dopamine Dimer I
Catalog No.:BCX0810
CAS No.:315188-82-0
- Monascorubrin
Catalog No.:BCX0811
CAS No.:13283-90-4
- 16-Hydroxyhexadecanoic acid
Catalog No.:BCX0812
CAS No.:506-13-8
- Xylotetraose
Catalog No.:BCX0813
CAS No.:22416-58-6
- Nepetalacton
Catalog No.:BCX0814
CAS No.:21651-62-7
- Kuwanon U
Catalog No.:BCX0815
CAS No.:123702-95-4
- Xylopentaose
Catalog No.:BCX0816
CAS No.:49694-20-4
- Hirudonucleodisulfide A
Catalog No.:BCX0817
CAS No.:1072789-37-7
- Xylohexaose
Catalog No.:BCX0818
CAS No.:49694-21-5
- Coreoside B
Catalog No.:BCX0819
CAS No.:1580464-83-0
- (25R)-26-O-β-D-Glucopyranosyl-22-hydroxy-5β-furost-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX0820
CAS No.:897386-27-5
Investigation of monacolin K, yellow pigments, and citrinin production capabilities of Monascus purpureus and Monascus ruber (Monascus pilosus).[Pubmed:37224553]
J Food Drug Anal. 2023 Mar 15;31(1):85-94.
Red mold rice (RMR) is a traditional Chinese medicine prepared using Monascus fermentation. Monascus ruber ( pilosus) and Monascus purpureus have a long history of use as food and medicine. As an economically important starter culture, the relationship between the taxonomy of Monascus and production capabilities of secondary metabolites is crucial for the Monascus food industry. In this study, monacolin K, Monascin, ankaflavin, and citrinin production by M. purpureus and M. ruber were genomically and chemically investigated. Our findings suggest that M. purpureus can produce Monascin and ankaflavin in a correlated manner, whereas M. ruber produces Monascin with minimum ankaflavin. M. purpureus is capable of producing citrinin; however, it is unlikely able to produce monacolin K. In contrast, M. ruber produces monacolin K, but not citrinin. We suggest that the current monacolin K content-related regulation of Monascus food should be revised, and labeling of Monascus species should be considered.
Fungal Pigments: Carotenoids, Riboflavin, and Polyketides with Diverse Applications.[Pubmed:37108908]
J Fungi (Basel). 2023 Apr 7;9(4):454.
Natural pigments and colorants have seen a substantial increase in use over the last few decades due to their eco-friendly and safe properties. Currently, customer preferences for more natural products are driving the substitution of natural pigments for synthetic colorants. Filamentous fungi, particularly ascomycetous fungi (Monascus, Fusarium, Penicillium, and Aspergillus), have been shown to produce secondary metabolites containing a wide variety of pigments, including beta-carotene, melanins, azaphilones, quinones, flavins, ankaflavin, Monascin, anthraquinone, and naphthoquinone. These pigments produce a variety of colors and tints, including yellow, orange, red, green, purple, brown, and blue. Additionally, these pigments have a broad spectrum of pharmacological activities, including immunomodulatory, anticancer, antioxidant, antibacterial, and antiproliferative activities. This review provides an in-depth overview of fungi gathered from diverse sources and lists several probable fungi capable of producing a variety of color hues. The second section discusses how to classify coloring compounds according to their chemical structure, characteristics, biosynthetic processes, application, and present state. Once again, we investigate the possibility of employing fungal polyketide pigments as food coloring, as well as the toxicity and carcinogenicity of particular pigments. This review explores how advanced technologies such as metabolic engineering and nanotechnology can be employed to overcome obstacles associated with the manufacture of mycotoxin-free, food-grade fungal pigments.
Neuroprotection by Nrf2 via modulating microglial phenotype and phagocytosis after intracerebral hemorrhage.[Pubmed:36852060]
Heliyon. 2023 Feb 16;9(2):e13777.
Activated microglia are divided into pro-inflammatory and anti-inflammatory functional states. In anti-inflammatory state, activated microglia contribute to phagocytosis, neural repair and anti-inflammation. Nrf2 as a major endogenous regulator in hematoma clearance after intracerebral hemorrhage (ICH) has received much attention. This study aims to investigate the mechanism underlying Nrf2-mediated regulation of microglial phenotype and phagocytosis in hematoma clearance after ICH. In vitro experiments, BV-2 cells were assigned to normal group and administration group (Nrf2-siRNA, Nrf2 agonists Monascin and Xuezhikang). In vivo experiments, mice were divided into 5 groups: sham, ICH + vehicle, ICH + Nrf2-/-, ICH + Monascin and ICH + Xuezhikang. In vitro and in vivo, 72 h after administration of Monascin and Xuezhikang, the expression of Nrf2, inflammatory-associated factors such as Trem1, TNF-alpha and CD80, anti-inflammatory, neural repair and phagocytic associated factors such as Trem2, CD206 and BDNF were analyzed by the Western blot method. In vitro, fluorescent latex beads or erythrocytes were uptaken by BV-2 cells in order to study microglial phagocytic ability. In vivo, hemoglobin levels reflect the hematoma volume. In this study, Nrf2 agonists (Monascin and Xuezhikang) upregulated the expression of Trem2, CD206 and BDNF while decreased the expression of Trem1, TNF-alpha and CD80 both in vivo and in vitro. At the same time, after Monascin and Xuezhikang treatment, the phagocytic capacity of microglia increased in vitro, neurological deficits improved and hematoma volume lessened in vivo. These results were reversed in the Nrf2-siRNA or the Nrf2-/- mice. All these results indicated that Nrf2 enhanced hematoma clearance and neural repair, improved neurological outcomes through enhancing microglial phagocytosis and alleviating neuroinflammation.
Supplementary effect of whey components on the monascin productivity of Monascus sp.[Pubmed:36732039]
J Sci Food Agric. 2023 Jun;103(8):4234-4241.
BACKGROUND: Monascus sp. has been used in fermented foods for centuries. It can synthesize yellow, red, and orange pigments as secondary metabolites. Here, we focused on yellow pigment Monascin, responsible for anti-inflammation and antidiabetic effects, and investigated whether whey could be a suitable substrate with or without rice powder for Monascin production using M. purpureus AHU 9085, M. pilosus NBRC 4520 and M. ruber NBRC 32318. RESULTS: The growth and Monascin production of the three Monascus strains were dependent on three liquid media consisting of whey and/or rice. All strains showed the best growth in a rice and whey mixed medium, in which M. ruber NBRC 32318 exhibited the highest total Monascin production. Subsequent investigation of the effects of whey components indicated that a mineral cocktail in whey was particularly effective in stimulating the Monascin production efficiency of M. ruber NBRC 32318. However, this recipe exhibited less stimulation, or even inhibition, for M. pilosus NBRC 4520 and M. purpureus AHU 9085, respectively. In terms of total Monascin production, rice with whey provided the highest amount due to growth promotion along with relatively high production efficiency. CONCLUSION: The effect of whey on growth and Monascin production was strongly dependent on the Monascus strains. Even a mineral cocktail in whey could regulate Monascin productivity in a strain-specific manner. Further studies are needed to elucidate the mechanism behind the diverse responses by the minerals in the production of Monascin from Monascus. (c) 2023 Society of Chemical Industry.
Chemical characterization and microencapsulation of extracellular fungal pigments.[Pubmed:36370157]
Appl Microbiol Biotechnol. 2022 Dec;106(24):8021-8034.
In this work, extracellular colored metabolites obtained from the filamentous fungi Talaromyces australis and Penicillium murcianum, isolated in the Andean-Patagonian native forests of Chile, were studied as prospect compounds to increase the sustainability of cosmetic products. The chemical and antioxidant properties of these natural pigments were characterized and strategies for their microencapsulation were also studied. UHPLC/MS-MS analyses indicated that the predominant metabolites detected in the cultures of P. murcianum were Monascin (m/z = 411.15) and monashexenone (m/z = 319.10), while athrorosin H (m/z = 458.20) and damnacanthal (m/z = 281.05) were detected in cultures of T. australis. ORAC tests revealed that P. murcianum's metabolites had the greatest antioxidant properties with values higher than 2000 mumol of trolox equivalents/g. The fungal metabolites were successfully microencapsulated by ionic gelation into structures made of 1.3% sodium alginate, 0.2% chitosan, and 0.07% hyaluronic acid. The microencapsulation process generated structures of 543.57 +/- 0.13 microm of mean diameter (d(50)) with an efficiency of 30% for P. murcianum, and 329.59 +/- 0.15 microm of mean diameter (d(50)) and 40% efficiency, for T. australis. The chemical and biological characterization show the biotechnological potential of these fungal species to obtain pigments with antioxidant activity that could be useful in the cosmetic industry. The encapsulation process enables the production of easy-to-handle dry powder from the fungal metabolites, which could be potentially marketed as a functional cosmetic ingredient. KEY POINTS: * The predominant fungal pigments were of azaphilone and anthraquinoid classes. * The fungal pigments showed high antioxidant activity by ORAC assay. * Fungal pigment microcapsules obtained by ionic gelation were characterized.
Monascin abrogates RANKL-mediated osteoclastogenesis in RAW264.7 cells via regulating MAPKs signaling pathways.[Pubmed:35910375]
Front Pharmacol. 2022 Jul 15;13:950122.
Osteoclasts (OCs) are multinucleated cells that play a major role in osteolytic diseases such as osteoporosis. Monascin (Ms) is one of the active substances in the traditional Chinese medicine red yeast rice. Studies have found that red yeast rice can maintain bone health. In this study, the anti-osteoclastogenesis effects of Ms on RANKL-induced RAW264.7 cells were assessed, and the underlying mechanism was investigated. Ms exhibited inhibitory effects on OC differentiation and formation in a dose-dependent manner and suppressed the bone-resorbing activity of mature OCs. Ms blocked OCs-typical genes (c-Fos, NFATc1, CSTK, MMP-9, TRAP, ITG-beta3, OSCAR and DC-STAMP). Furthermore, Ms treatment considerably inhibited the activation of MAPKs, JNK and p38. Taken together, Ms suppresses RANKL-induced osteoclastogenesis of RAW264.7 cells by restraining MAPKs signaling pathways and is a potential therapeutic option as a novel OC inhibitor to mitigate bone erosion.
A new endophyte Monascus ruber SRZ112 as an efficient production platform of natural pigments using agro-industrial wastes.[Pubmed:35871189]
Sci Rep. 2022 Jul 23;12(1):12611.
A number of biopigment applications in various industrial sectors are gaining importance due to the growing consumer interest in their natural origin. Thus, this work was conducted to valorize endophytic fungi as an efficient production platform for natural pigments. A promising strain isolated from leaves of Origanum majorana was identified as Monascus ruber SRZ112 produced several types of pigments. The nature of the pigments, mainly rubropunctamine, Monascin, ankaflavin, rubropunctatin, and monascorubrin in the fungal extract was studied by LC/ESI-MS/MS analyses. As a first step towards developing an efficient production of red pigments, the suitability of seven types of agro-industrial waste was evaluated. The highest yield of red pigments was obtained using potato peel moistened with mineral salt broth as a culture medium. To increase yield of red pigments, favourable culture conditions including incubation temperature, incubation period, pH of moistening agent, inoculum concentration, substrate weight and moisture level were evaluated. Additionally, yield of red pigments was intensified after the exposure of M. ruber SRZ112 spores to 1.00 KGy gamma rays. The final yield was improved by a 22.12-fold increase from 23.55 to 3351.87 AU g(-1). The anticancer and antioxidant properties of the pigment's extract from the fungal culture were also studied. The obtained data indicated activity of the extract against human breast cancer cell lines with no significant cytotoxicity against normal cell lines. The extract also showed a free radical scavenging potential. This is the first report, to our knowledge, on the isolation of the endophytic M. ruber SRZ112 strain with the successful production of natural pigments under solid-state fermentation using potato peel as a substrate.
Analysis of the differences in the chemical composition of monascus rice and highland barley monascus.[Pubmed:35723016]
Food Funct. 2022 Jul 4;13(13):7000-7019.
Monascus rice (MR) and highland barley monascus (HBM), the monascus fermented products, are applied in food and medicine to reduce cholesterol and promote digestion. Due to the fermentation substrates, their compositions are different. However, the exact differences have not been reported to date. By UPLC-Q-Orbitrap HRMS analysis, multiple components of twenty batches of MR and HBM samples were identified. In total, 100 components were confirmed (e.g., monacolins, pigments, decalin derivatives, amino acids). Then, principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were used to filter the components of MR and HBM. In the PCA model, 88.1% of the total variance was uncovered. The OPLS-DA model showed better discrimination between MR and HBM, and the values of R(2)X, R(2)Y, and Q(2) were 0.837, 0.996, and 0.956, respectively. Based on the value of the variable importance in projection (VIP) and the result of the t-test, 424 components (VIP > 1, p < 0.05) were acquired. Finally, 11 differential components were selected as the characteristic compounds to discriminate between MR and HBM: the content of 9 monacolins (3-hydroxy-3,5-dihydrodehydromonacolin K, monacolin K, dehydromonacolin K, dehydromonacolin J hydroxy acid, monacophenyl, dihydromonacolin J hydroxy acid, monacolin L, dehydromonacolin J, and monacolin R) in HBM was higher than the content in MR, but the content of 2 pigments (ankaflavin and Monascin) was lower in HBM and higher in MR. The findings revealed the similarities and differences in the chemical composition between MR and HBM, which is expected to provide a basis for quality control of HBM.
Monascin and ankaflavin-Biosynthesis from Monascus purpureus, production methods, pharmacological properties: A review.[Pubmed:35353924]
Biotechnol Appl Biochem. 2023 Feb;70(1):137-147.
Monascus purpureus copiously yields beneficial secondary metabolites , including Monascus pigments, which are broadly used as food additives, as a nitrite substitute in meat products, and as a colorant in the food industry. Monascus yellow pigments (Monascin and ankaflavin) have shown potential antidiabetic, antibacterial, anti-inflammatory, antidepressant, antibiotic, anticancer, and antiobesity activities. Cosmetic and textile industries are other areas where it has established its potential as a dye. This paper reviews the production methods of Monascus yellow pigments, biosynthesis of Monascus pigments from M. purpureus, factors affecting yellow pigment production during fermentation, and the pharmacological properties of Monascin and ankaflavin.
Toxicological evaluation of the red mold rice extract, ANKASCIN 568-R: 13-week chronic toxicity, and genotoxicity studies.[Pubmed:35284245]
Toxicol Rep. 2022 Feb 22;9:356-365.
ANKASCIN 568-R is an extract derived from red mold rice (RMR) fermented using Monascus purpureus NTU 568. RMR fermented using M. purpureus NTU 568 prevents cardiovascular diseases and decreases blood lipid levels. This study evaluates the safety of ANKASCIN 568-R, since it has not determined yet. After daily oral ANKASCIN 568-R for 13 consecutive weeks, we evaluated the toxicity tolerance of Sprague-Dawley rats and performed dose formulation analysis on Monascin and ankaflavin. The dose formulation analysis showed that ANKASCIN 568-R concentrations were lower than the target concentration and out of range ( +/- 15%) at week 8 and on the last dosing day for both Monascin (all dose groups) and ankaflavin at the 100 mg/kg dose. The lowest reported concentrations for the low, middle, and high dose formulations were 34.7, 115.2, and 398.1 mg/mL, respectively. We also evaluated the genotoxicity of ANKASCIN 568-R and showed no genotoxicity potential at all ANKASCIN 568-R doses investigated. The no observed adverse effect level of ANKASCIN 568-R was determined to be 796.2 mg/kg/day. This study revealed the first toxicity evaluation data of ANKASICN 568-R, and the data demonstrated ANKASICN 568-R was safe and can be used in daily life.
Combination of (1)H and (13)C NMR for quantitative analysis of the orange pigments produced by Monascus kaoliang KB9.[Pubmed:34933631]
Nat Prod Res. 2023 Apr;37(8):1406-1409.
Two orange pigments, rubropunctatin (1) and monascorubrin (2), along with the yellow pigments, Monascin (3) and ankaflavin (4), were isolated from M. kaoliang KB9-fermented rice, also known as red yeast rice. The orange pigments exhibit a broad spectrum of biological activities and appeared to be the major components of this fermented rice. In this work, quantitative (1)H NMR (qHNMR) and (13)C NMR experiments were used to determine the amounts of the two orange pigments in a crude extract in which most of the (1)H NMR signals of the two compounds were indistinguishable. The quantitative values obtained by NMR techniques were found to be similar to those obtained by HPLC. Thus, the combined qHNMR with (13)C experiment described in this work could be further developed to quantifying Monascus pigments or other invaluable natural products when qHNMR alone is insufficient for quantitative analysis.
Monascin and Ankaflavin of Monascus purpureus Prevent Alcoholic Liver Disease through Regulating AMPK-Mediated Lipid Metabolism and Enhancing Both Anti-Inflammatory and Anti-Oxidative Systems.[Pubmed:34684882]
Molecules. 2021 Oct 18;26(20):6301.
Alcohol metabolism causes an excessive accumulation of liver lipids and inflammation, resulting in liver damage. The yellow pigments Monascin (MS) and ankaflavin (AK) of Monascus purpureus-fermented rice were proven to regulate ethanol-induced damage in HepG2 cells, but the complete anti-inflammatory and anti-fatty liver mechanisms in the animal model are still unclear. This study explored the roles of MS and AK in improving alcoholic liver injury. MS and AK were simultaneously fed to evaluate their effects and mechanisms in C57BL/6J mice fed the Lieber-DeCarli liquid alcohol diet for 6 weeks. The results indicated that MS and AK significantly reduced the serum aspartate aminotransferase and alanine aminotransferase activity, as well as the total liver cholesterol and triglyceride levels. The histopathological results indicated that MS and AK prevented lipid accumulation in the liver. MS and AK effectively enhanced the activity of antioxidant enzymes and reduced the degree of lipid peroxidation; AK was particularly effective and exhibited a superior preventive effect against alcoholic liver injury and fatty liver. In addition to inhibiting the phosphorylation of the MAPK family, MS and AK directly reduced TNF-alpha, IL-6, and IL-1beta levels, thereby reducing NF-kappaB and its downstream iNOS and COX-2 expressions, as well as increasing PPAR-gamma, Nrf-2, and HO-1 expressions to prevent liver damage. MS and AK also directly reduced TNF-alpha, IL-6, and IL-1beta expression, thereby reducing the production of NF-kappaB and its downstream iNOS and COX-2, and increasing PPAR-gamma, Nrf-2, and HO-1 expressions, preventing alcohol damage to the liver.
Comparative Study on the Antioxidant Activity of Monascus Yellow Pigments From Two Different Types of Hongqu-Functional Qu and Coloring Qu.[Pubmed:34408740]
Front Microbiol. 2021 Aug 2;12:715295.
This study is the first to investigate the difference in the composition of Monascus azaphilone pigments (MonAzPs) between functional Qu (FQ) and coloring Qu (CQ) and analyze their relationships with antioxidant activity. The composition of key active components and antioxidant activity of the ethanol extracts of FQ and CQ were analyzed by Uv-vis, HPLC, and chemical antioxidant tests. The composition of MonAzPs of the ethanol extracts was further analyzed by HPLC-MS. Seven Monascus yellow pigments (MYPs) with high abundance were successfully purified for the antioxidation evaluation in vitro and in the cell. Correlation analysis between the metabolites and the antioxidant activity of Hongqu indicated that MonAzPs might play an essential role in the antioxidant activity (r > 0.80). By contrast, the monacolin K (MK), polysaccharide, ergosterol, and gamma-aminobutyric acid (GABA) were not significantly correlated with the antioxidant activity. Orthogonal partial least squares discriminant analysis (OPLS-DA) based on the composition of MonAzPs revealed that the abundance of MYPs is significantly different between FQ and CQ (P < 0.05 and VIP > 1.0). Seven MYPs (monasfluore A, monaphilone B, monascuspilion, Monascin, monaphilone A, ankaflavin, and new yellow pigment) with high abundance were successfully purified for the antioxidation evaluation. Chemical antioxidant tests revealed that the antioxidant activities of monaphilone A, ankaflavin, and new yellow pigment only from CQ were significantly more potent than monasfluore A and monascuspilion only separated from FQ. The cellular antioxidant assay (CAA) showed that the new yellow pigment had the best antioxidant activity (quercetin equivalent 7.23 muM), followed by monasfluore A and monaphilone B, all of which were significantly better than Monascin and ankaflavin, the two most frequently reported MYPs. Research on the structure-activity relationship demonstrated that alterations of the hydroxyl that occurred on C-3' or C-11 obviously affected the antioxidant activities of MYPs. Our findings provide evidence that MYPs may be the key active components for CQ to have a more potent antioxidant capacity than FQ. The alterations of the hydroxyl that occurred on C-3' or C-11 obviously affected the antioxidant activities of MYPs.


