MonascorubrinCAS# 13283-90-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 13283-90-4 | SDF | Download SDF |
| PubChem ID | 12118084.0 | Appearance | Powder |
| Formula | C23H26O5 | M.Wt | 382.45 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
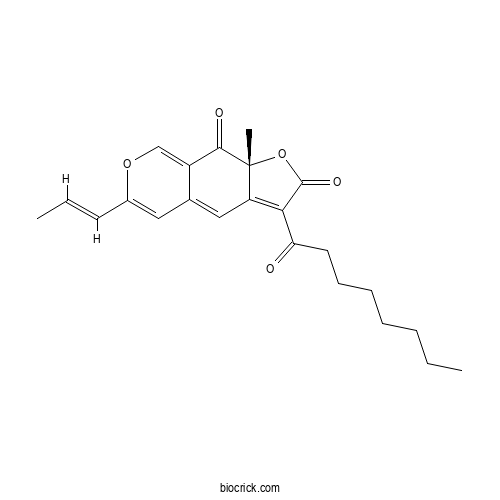
| Chemical Name | (9aR)-9a-methyl-3-octanoyl-6-[(E)-prop-1-enyl]furo[3,2-g]isochromene-2,9-dione | ||
| SMILES | CCCCCCCC(=O)C1=C2C=C3C=C(OC=C3C(=O)C2(OC1=O)C)C=CC | ||
| Standard InChIKey | IIPVSGPTPPURBD-HAOIVFDCSA-N | ||
| Standard InChI | InChI=1S/C23H26O5/c1-4-6-7-8-9-11-19(24)20-18-13-15-12-16(10-5-2)27-14-17(15)21(25)23(18,3)28-22(20)26/h5,10,12-14H,4,6-9,11H2,1-3H3/b10-5+/t23-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Monascorubrin Dilution Calculator

Monascorubrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6147 mL | 13.0736 mL | 26.1472 mL | 52.2944 mL | 65.368 mL |
| 5 mM | 0.5229 mL | 2.6147 mL | 5.2294 mL | 10.4589 mL | 13.0736 mL |
| 10 mM | 0.2615 mL | 1.3074 mL | 2.6147 mL | 5.2294 mL | 6.5368 mL |
| 50 mM | 0.0523 mL | 0.2615 mL | 0.5229 mL | 1.0459 mL | 1.3074 mL |
| 100 mM | 0.0261 mL | 0.1307 mL | 0.2615 mL | 0.5229 mL | 0.6537 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetyl Dopamine Dimer I
Catalog No.:BCX0810
CAS No.:315188-82-0
- guan-fu base I
Catalog No.:BCX0809
CAS No.:110225-59-7
- Monascin
Catalog No.:BCX0808
CAS No.:21516-68-7
- Thymoquinone
Catalog No.:BCX0807
CAS No.:490-91-5
- Neoeuonymine
Catalog No.:BCX0806
CAS No.:33510-25-7
- Cortodoxone
Catalog No.:BCX0805
CAS No.:152-58-9
- Cortisone
Catalog No.:BCX0804
CAS No.:53-06-5
- Quercetin 7-O-glucuronide
Catalog No.:BCX0803
CAS No.:38934-20-2
- Coreopsin
Catalog No.:BCX0802
CAS No.:499-29-6
- Harmalan
Catalog No.:BCX0801
CAS No.:525-41-7
- Prosaikogenin G
Catalog No.:BCX0800
CAS No.:99365-23-8
- Pterin-6-carboxylic acid
Catalog No.:BCX0799
CAS No.:948-60-7
- 16-Hydroxyhexadecanoic acid
Catalog No.:BCX0812
CAS No.:506-13-8
- Xylotetraose
Catalog No.:BCX0813
CAS No.:22416-58-6
- Nepetalacton
Catalog No.:BCX0814
CAS No.:21651-62-7
- Kuwanon U
Catalog No.:BCX0815
CAS No.:123702-95-4
- Xylopentaose
Catalog No.:BCX0816
CAS No.:49694-20-4
- Hirudonucleodisulfide A
Catalog No.:BCX0817
CAS No.:1072789-37-7
- Xylohexaose
Catalog No.:BCX0818
CAS No.:49694-21-5
- Coreoside B
Catalog No.:BCX0819
CAS No.:1580464-83-0
- (25R)-26-O-β-D-Glucopyranosyl-22-hydroxy-5β-furost-3β,26-diol-3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside
Catalog No.:BCX0820
CAS No.:897386-27-5
- Tribuloside
Catalog No.:BCX0821
CAS No.:22153-44-2
- 6''- methyl glycyrrhizinate
Catalog No.:BCX0822
CAS No.:1186016-30-7
- Picfeltarraegenin I
Catalog No.:BCX0823
CAS No.:82145-63-9
Mannitol improves Monascus pigment biosynthesis with rice bran as a substrate in Monascus purpureus.[Pubmed:38156009]
Front Microbiol. 2023 Dec 13;14:1300461.
To reduce the production cost of Monascus pigments (MPs), the utilization of rice bran (RB), an agricultural waste product, as a substrate in submerged fermentation was conducted in this study. To improve MP production, different nutritional ingredients including mannitol (Man), NH(4)NO(3) (AN), ZnSO(4) (Zn), and optimization (Opti), which was a synthesis of the three above ones, were added in rice bran (RB) medium. The yields of MPs, pigment constituents, and growth and development of Monascus purpureus M9 were investigated in this study. Man had the maximum color value of 3,532 U/g, which was 18.69 times more than that of RB and reached up to 76.65% of the value of rice (Rice) fermentation. Man significantly increased the production of two orange pigments, Monascorubrin and rubropunctatin, of which the yields were 69.49 and 95.36% of the counterpart of Rice. The biomass and colony diameter of Opti presented the maximum value among different groups. AN and RB induced more asexual spore formation, whereas Opti and Man promoted sexual spore production. Comparative transcriptomic analysis showed that different nutritional ingredients led to changes in pigment production, promoting the growth and development of M. purpureus M9 through the regulation of related gene expression. Man and Opti improved MP production by regulating the primary metabolism, including the Embden-Meyerhof pathway (EMP), the pentose phosphate (PP) pathway, the tricarboxylic (TCA) cycle, fatty acid degradation (FAD), fatty acid biosynthesis (FAB), amino acid metabolism (AAM), and fructose and mannose metabolism (FMM), to provide the precursors (acetyl-CoA and malonyl-CoA) for MP biosynthesis. This study presents a low-cost method for increasing MP production and explains the molecular mechanisms of different nutritional ingredients for enhancing MP biosynthesis.
Precursor-directed production of water-soluble red Monascus pigments with high thermal stability via azaphilic addition reaction-based semi-synthesis.[Pubmed:38144809]
Food Chem X. 2023 Oct 10;20:100940.
Red Monascus pigments (MPs) are a large group of polyketides from the fungus Monascus which have been widely used as food colorants. In this study, a variety of red MPs congeners were prepared to explore promising water-soluble candidates for application in liquid food formulations. The results showed that by combining the two-stage, low-pH fermentation strategy with a downstream purification step of fractional crystallization, precursors of red MPs, namely Monascorubrin and rubropunctatin, were obtained with a purity of 91.9%. Then, via the azaphilic addition reaction, 18 types of red MPs congeners carrying different amino acid moieties (MPs-aa) were semi-synthesized. Compared to rubropunctamine and monascorubramine, the water solubility, pH and thermal stability of MPs-aa were improved greatly. MPs-His, MPs-Phe, MPs-Tyr and MPs-Trp were identified to be the most resistant to pasteurization. These findings provide water-soluble red MPs candidates with high thermal stability and an attractive approach for their large scale production.
Preparation of Thin Film Composite (TFC) Membrane with DESPs Interlayer and Its Forward Osmosis (FO) Performance for Organic Solvent Recovery.[Pubmed:37505054]
Membranes (Basel). 2023 Jul 24;13(7):688.
To explore the application of forward osmosis (FO) technology in the organic solvent recovery field, we prepared a new solvent-resistant triple layer thin film composite (TFC) membrane on the PI (polyimide) substrate. The deep eutectic supramolecular polymers (DESPs) interlayer was constructed on the substrate to improve the separation performance and solvent resistance. DESPs interlayer was formed by mixing and heating with cyclodextrin as the hydrogen bond acceptor and L-malic acid as the hydrogen bond donor. The chemical changes, surface property and morphology of the composite membrane with DESPs interlayer were characterized. The separation performance and stability of the triple layer composite membrane in organic solvent FO were studied. For the Monascorubrin-ethanol system, the permeation flux of TFC/DESPs5-PI membrane could reach 9.51 LMH while the rejection rate of Monascorubrin was 98.4% (1.0 M LiCl/ethanol as draw solution), which was better than the pristine membrane. Therefore, this solvent-resistant triple layer composite FO membrane has good potential for the recovery of organic solvents.
A new endophyte Monascus ruber SRZ112 as an efficient production platform of natural pigments using agro-industrial wastes.[Pubmed:35871189]
Sci Rep. 2022 Jul 23;12(1):12611.
A number of biopigment applications in various industrial sectors are gaining importance due to the growing consumer interest in their natural origin. Thus, this work was conducted to valorize endophytic fungi as an efficient production platform for natural pigments. A promising strain isolated from leaves of Origanum majorana was identified as Monascus ruber SRZ112 produced several types of pigments. The nature of the pigments, mainly rubropunctamine, monascin, ankaflavin, rubropunctatin, and Monascorubrin in the fungal extract was studied by LC/ESI-MS/MS analyses. As a first step towards developing an efficient production of red pigments, the suitability of seven types of agro-industrial waste was evaluated. The highest yield of red pigments was obtained using potato peel moistened with mineral salt broth as a culture medium. To increase yield of red pigments, favourable culture conditions including incubation temperature, incubation period, pH of moistening agent, inoculum concentration, substrate weight and moisture level were evaluated. Additionally, yield of red pigments was intensified after the exposure of M. ruber SRZ112 spores to 1.00 KGy gamma rays. The final yield was improved by a 22.12-fold increase from 23.55 to 3351.87 AU g(-1). The anticancer and antioxidant properties of the pigment's extract from the fungal culture were also studied. The obtained data indicated activity of the extract against human breast cancer cell lines with no significant cytotoxicity against normal cell lines. The extract also showed a free radical scavenging potential. This is the first report, to our knowledge, on the isolation of the endophytic M. ruber SRZ112 strain with the successful production of natural pigments under solid-state fermentation using potato peel as a substrate.
Red yeast rice dietary intervention reduces oxidative stress-related inflammation and improves intestinal microbiota.[Pubmed:35621018]
Food Funct. 2022 Jun 20;13(12):6583-6595.
Inflammation and oxidative stress play key roles in the aging process, while red yeast rice (RYR), a traditional Chinese fermented food, has anti-oxidant and anti-inflammatory effects. To understand the anti-aging function of RYR in vivo, this study established a D-galactose-induced aging mouse model to verify the positive effects of RYR dietary intervention on aging and explore the related underlying mechanism. Eight weeks of RYR dietary intervention was shown to have a significant inhibitory effect on cognitive decline and hippocampal damage. The molecular mechanistic studies showed that the anti-aging effects of RYR were achieved by (i) improving the oxidative stress-related damage (increasing SOD, CAT, and GSH, and reducing MDA), (ii) regulating the NF-kappaB inflammation pathway induced by oxidative stress (decreasing the pro-inflammatory cytokines IL-6, TNF-alpha, IFN-gamma, iNOs, and IL-1beta, increasing the anti-inflammatory cytokine IL-10, and decreasing the expression of the NF-kappaB protein), (iii) slowing down apoptosis caused by oxidative stress (reducing the expression of P21 and P53), (iv) restoring the abundance of Lactobacillus, Lachnospiraceae and Rikenellaceae downregulated by D-galactose, and (v) reducing the abundance of Akkermansia and Helicobacter enriched by D-galactose. Mass spectrometry revealed orange pigments (rubropunctatin and Monascorubrin) as the main antioxidant components in RYR, which might play key roles in aging inhibition. This study provides theoretical support for the wide application of orange pigments as an antioxidant dietary supplement.
Solid-state co-culture fermentation of simulated food waste with filamentous fungi for production of bio-pigments.[Pubmed:35608668]
Appl Microbiol Biotechnol. 2022 Jun;106(11):4029-4039.
The use of waste stream residues as feedstock for material production simultaneously helps reduce dependence on fossil-based resources and to shift toward a circular economy. This study explores the conversion of food waste into valuable chemicals, namely, bio-pigments. Here, a simulated food waste feedstock was converted into pigments via solid-state fermentation with the filamentous fungus Talaromyces albobiverticillius (NRRL 2120). Pigments including Monascorubrin, rubropunctatin, and 7-(2-hydroxyethyl)-monascorubramine were identified as products of the fermentation via ultra-performance liquid chromatography coupled with quadrupole-time-of-flight electrospray ionization mass spectrometry. Pigments were obtained at concentrations of 32.5, 20.9, and 22.4 AU/gram dry substrate for pigments absorbing at 400, 475, and 500 nm, respectively. Pigment production was further enhanced by co-culturing T. albobiverticillius with Trichoderma reesei (NRRL 3652), and ultimately yielded 63.8, 35.6, and 43.6 AU/gds at the same respective wavelengths. This represents the highest reported production of pigments via solid-state fermentation of a non-supplemented waste stream feedstock. KEY POINTS: * Simulated food waste underwent solid-state fermentation via filamentous fungi. * Bio-pigments were obtained from fermentation of the simulated food waste. * Co-culturing multiple fungal species substantially improved pigment production.
Chemical Profiling, Bioactivity Evaluation and the Discovery of a Novel Biopigment Produced by Penicillium purpurogenum CBS 113139.[Pubmed:35011300]
Molecules. 2021 Dec 23;27(1):69.
Biobased pigments are environmentally friendly alternatives to synthetic variants with an increased market demand. Production of pigments via fermentation is a promising process, yet optimization of the production yield and rate is crucial. Herein, we evaluated the potential of Penicillium purpurogenum to produce biobased pigments. Optimum sugar concentration was 30 g/L and optimum C:N ratio was 36:1 resulting in the production of 4.1-4.5 AU (namely Pigment Complex A). Supplementation with ammonium nitrate resulted in the production of 4.1-4.9 AU (namely Pigment Complex B). Pigments showed excellent pH stability. The major biopigments in Pigment Complex A were N-threonyl-rubropunctamin or the acid form of PP-R (red pigment), N-GABA-PP-V (violet pigment), PP-O (orange pigment) and Monascorubrin. In Pigment Complex B, a novel biopigment annotated as N-GLA-PP-V was identified. Its basic structure contains a polyketide azaphilone with the same carboxyl-monascorubramine base structure as PP-V (violet pigment) and gamma-carboxyglutamic acid (GLA). The pigments were not cytotoxic up to 250 mug/mL.
Combination of (1)H and (13)C NMR for quantitative analysis of the orange pigments produced by Monascus kaoliang KB9.[Pubmed:34933631]
Nat Prod Res. 2023 Apr;37(8):1406-1409.
Two orange pigments, rubropunctatin (1) and Monascorubrin (2), along with the yellow pigments, monascin (3) and ankaflavin (4), were isolated from M. kaoliang KB9-fermented rice, also known as red yeast rice. The orange pigments exhibit a broad spectrum of biological activities and appeared to be the major components of this fermented rice. In this work, quantitative (1)H NMR (qHNMR) and (13)C NMR experiments were used to determine the amounts of the two orange pigments in a crude extract in which most of the (1)H NMR signals of the two compounds were indistinguishable. The quantitative values obtained by NMR techniques were found to be similar to those obtained by HPLC. Thus, the combined qHNMR with (13)C experiment described in this work could be further developed to quantifying Monascus pigments or other invaluable natural products when qHNMR alone is insufficient for quantitative analysis.
A facile macroporous resin-based method for separation of yellow and orange Monascus pigments.[Pubmed:33936846]
Food Sci Biotechnol. 2021 Mar 8;30(4):545-553.
The yellow Monascus pigments (YMPs) named monascin and ankaflavin and the orange Monascus pigments (OMPs) named rubropunctatin and Monascorubrin are two groups of bioactive components in a mixture state in the Monascus fermented products. In order to separate these two groups of bioactive pigments, a facile macroporous resin-based method was developed. The weak-polar resin CAD-40 was selected from the seven tested macroporous resins as it revealed better properties for the adsorption and desorption of the YMPs and OMPs. Then, CAD-40 resin was used for column-chromatographic separation. After eluted by 4 bed volumes of ethanol, the yellow group (monascin and ankaflavin) and the orange group (rubropunctatin and Monascorubrin) were successfully separated and purified, with an increased content from 49.3% and 44.2% in the crude pigment extract to 85.2% and 83.0% in the final products, respectively. This method would be helpful for the large-scale separation and purification of Monascus pigment products with specific bioactivity.
Ammonium nitrate regulated the color characteristic changes of pigments in Monascus purpureus M9.[Pubmed:33398480]
AMB Express. 2021 Jan 4;11(1):3.
Monascus pigments (MPs) with different color characteristics, produced by submerged fermentation of Monascus purpureus M9, have potential application in food industry. In the present study, the effects and regulatory mechanisms of ammonium nitrate (AN) on the color characteristics of MPs were investigated. The concentration of intracellular pigments was significantly decreased when subjected to AN. The hue and lightness value indicated AN altered the total pigments appearance from original red to orange. The HPLC analysis for six major components of MPs showed that the production of rubropunctatin or Monascorubrin, was significantly reduced to the undetectable level, whereas the yields of monascin, ankaflavin, rubropunctamine and monascorubramine, were apparently increased with AN supplement. To be noted, via real-time quantitative PCR strategy, the expression level of mppG, closely relative to orange pigments biosynthesis, was significantly down-regulated. However, the expression of mppE, involved in yellow pigments pathway, was up-regulated. Moreover, the broth pH value was dropped to 2.5-3.5 in the fermentation process resulted from AN treatment, along with the increased extracellular polysaccharide biosynthesis. Taken together, the change of MPs categories and amounts by AN might be the driving force for the color characteristics variation in M. purpureus M9. The present study provided useful data for producing MPs with different compositions and modified color characteristics.
Effects of mrpigG on Development and Secondary Metabolism of Monascus ruber M7.[Pubmed:32872515]
J Fungi (Basel). 2020 Aug 29;6(3):156.
Monascus pigments (MPs) have been used as food colorants for several centuries in Asian countries and are now used throughout the world via Asian catering. The MP biosynthetic pathway has been well-illustrated, but the functions of a few genes, including mrpigG, in the MP gene cluster are still unclear. In the current study, in order to investigate the function of mrpigG in M. ruber M7, gene deletion (DeltamrpigG), complementation (DeltamrpigG::mrpigG) and overexpression (M7::PtrpC-mrpigG) mutants were successfully obtained. The morphologies and biomasses, as well as the MP and citrinin production, of these mutants were analyzed. The results revealed that the disruption, complementation and overexpression of mrpigG showed no apparent defects in morphology, biomass or citrinin production (except MP production) in DeltamrpigG compared with M. ruber M7. Although the MP profiles of DeltamrpigG and M. ruber M7 were almost the same-with both having four yellow pigments, two orange pigments (OPs) and two red pigments (RPs)-their yields were decreased in DeltamrpigG to a certain extent. Particularly, the content of rubropunctatin (an OP) and its derivative rubropunctamine (an RP) in DeltamrpigG, both of which have a five-carbon side chain, accounted for 57.7%, and 22.3% of those in M. ruber M7. On the other hand, Monascorubrin (an OP) and its derivative monascorubramine (an RP), both of which have a seven-carbon side chain, were increased by 1.15 and 2.55 times, respectively, in DeltamrpigG compared with M. ruber M7. These results suggest that the MrPigG protein may preferentially catalyze the biosynthesis of MPs with a five-carbon side chain.
Transfigured Morphology and Ameliorated Production of Six Monascus Pigments by Acetate Species Supplementation in Monascus ruber M7.[Pubmed:31936171]
Microorganisms. 2020 Jan 7;8(1):81.
Monascus species have been used for the production of many industrially and medically important metabolites, most of which are polyketides produced by the action of polyketide synthases that use acetyl-CoA and malonyl-CoA as precursors, and some of them are derived from acetate. In this study the effects of acetic acid, and two kinds of acetates, sodium acetate and ammonium acetate at different concentrations (0.1%, 0.25% and 0.5%) on the morphologies, biomasses, and six major Monascus pigments (MPs) of M. ruber M7 were investigated when M7 strain was cultured on potato dextrose agar (PDA) at 28 degrees C for 4, 8, 12 days. The results showed that all of the added acetate species significantly affected eight above-mentioned parameters. In regard to morphologies, generally the colonies transformed from a big orange fleecy ones to a small compact reddish ones, or a tightly-packed orange ones without dispersed mycelia with the increase of additives concentration. About the biomass, addition of ammonium acetate at 0.1% increased the biomass of M. ruber M7. With respect to six MPs, all acetate species can enhance pigment production, and ammonium acetate has the most significant impacts. Production of monascin and ankaflavin had the highest increase of 11.7-fold and 14.2-fold in extracellular contents at the 8th day when 0.1% ammonium acetate was supplemented into PDA. Intracellular rubropunctatin and Monascorubrin contents gained 9.6 and 6.46-fold at the 8th day, when 0.1% ammonium acetate was added into PDA. And the extracellular contents of rubropunctamine and monascorubramine were raised by 1865 and 4100-fold at the 4th day when M7 grew on PDA with 0.5% ammonium acetate.
Safety efficacy and chemical profiling of water-soluble Talaromyces purpureogenus CFRM02 pigment.[Pubmed:31771918]
Food Chem. 2020 Apr 25;310:125869.
Talaromyces purpureogenus CFRM02 pigments are non-toxic to Artemia franciscana. Further, in acute toxicity study, single dose (50, 300, 1000 and 2000 mg/kg body weight) pigment was administered to female Wistar rats. After 14 days, no evidence of adverse effect on body weight, mortality and clinical signs were observed. Similarly, 28 days sub-acute studies (250-1000 mg/kg body weight) showed no significant changes in food intake, body weight gain and relative weight of vital organs. No signs of toxicity on biochemical, hematological parameters. Histopathological examination of the liver and kidney were normal. There were no marked changes in any of the serum enzymes activities. There were no significant changes in treated and control group (acute and sub-acute). The HRMS data revealed the identification of purpuride, PP-O, PP-R, pentalsamonin, puractin-A, arginine-Monascorubrin, purpurquinone-A, ankaflavin, purpactin-C. These results confirmed safety efficacy of T. purpureogenus CFRM02 pigment and suggested applications in food and nutraceuticals.
Effect of initial pH, different nitrogen sources, and cultivation time on the production of yellow or orange Monascus purpureus pigments and the mycotoxin citrinin.[Pubmed:31763000]
Food Sci Nutr. 2019 Sep 27;7(11):3494-3500.
Monascus purpureus was grown in submerged liquid culture using ammonium sulfate, sodium nitrate, and peptone as nitrogen sources while initial medium pH was adjusted to 2.5, 5.5, 6.5, or 8.0. The combined effect of culture pH and nitrogen source on the biosynthesis of yellow (ankaflavin and monascin) and orange (rubropunctatin and Monascorubrin) pigments, plus the mycotoxin citrinin, was evaluated chromatographically. Optimum cultivation conditions, that is, initial pH 2.5 and 8.8 g/L peptone as a nitrogen source, resulted in high levels of production of yellow and orange pigments (sum of pigment concentration 1,138 mg/L) and negligible citrinin concentration (2 mg/L).
Acidic conditions induce the accumulation of orange Monascus pigments during liquid-state fermentation of Monascus ruber M7.[Pubmed:31501941]
Appl Microbiol Biotechnol. 2019 Oct;103(20):8393-8402.
The influence of pH on the biosynthesis of orange Monascus pigments (OMPs) in Monascus ruber M7 was investigated. Under acidic fermentation conditions, pigment mixtures predominantly rich in OMPs were obtained. HPLC analysis revealed the presence of four orange components (O1-O4) and four yellow components (Y1-Y4) in the mixtures, and the dominant ones were O1 and O3, which accounted for 56.0% to 75.9% of the total pigments in the pH range 3-6. Subsequently, O1 and O3 were identified by LC-DAD-ESI/MS as Rubropunctatin and Monascorubrin, respectively. The yield of OMPs was observed to be inversely dependent on pH. At pH 3, large amounts of OMPs with high purity (79.1%) were accumulated. A real-time quantitative PCR analysis revealed that the expression of genes related to the biosynthesis of OMPs in M. ruber M7 was upregulated at acidic pH as compared to neutral pH, and the variation in the level of expression of these genes with pH was consistent with the production of OMPs. These results indicated that the large accumulation of OMPs under acidic condition involved the acidic pH-induced transcription of genes related to the biosynthesis of OMPs. These results would contribute towards the development of an efficient technology for large-scale production of OMPs.


