CortodoxoneCAS# 152-58-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 152-58-9 | SDF | Download SDF |
| PubChem ID | 440707.0 | Appearance | Powder |
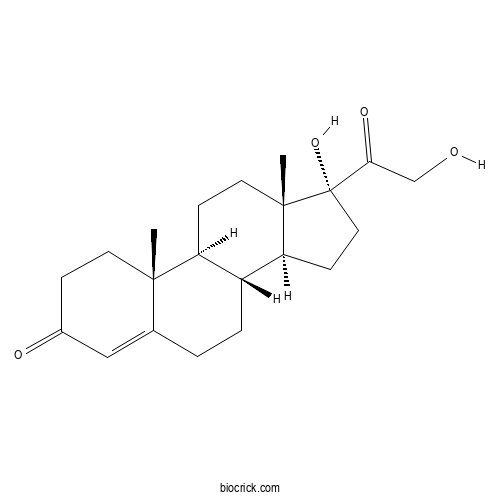
| Formula | C21H30O4 | M.Wt | 346.46 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8R,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthren-3-one | ||
| SMILES | CC12CCC(=O)C=C1CCC3C2CCC4(C3CCC4(C(=O)CO)O)C | ||
| Standard InChIKey | WHBHBVVOGNECLV-OBQKJFGGSA-N | ||
| Standard InChI | InChI=1S/C21H30O4/c1-19-8-5-14(23)11-13(19)3-4-15-16(19)6-9-20(2)17(15)7-10-21(20,25)18(24)12-22/h11,15-17,22,25H,3-10,12H2,1-2H3/t15-,16+,17+,19+,20+,21+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Cortodoxone Dilution Calculator

Cortodoxone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8863 mL | 14.4317 mL | 28.8634 mL | 57.7267 mL | 72.1584 mL |
| 5 mM | 0.5773 mL | 2.8863 mL | 5.7727 mL | 11.5453 mL | 14.4317 mL |
| 10 mM | 0.2886 mL | 1.4432 mL | 2.8863 mL | 5.7727 mL | 7.2158 mL |
| 50 mM | 0.0577 mL | 0.2886 mL | 0.5773 mL | 1.1545 mL | 1.4432 mL |
| 100 mM | 0.0289 mL | 0.1443 mL | 0.2886 mL | 0.5773 mL | 0.7216 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cortisone
Catalog No.:BCX0804
CAS No.:53-06-5
- Quercetin 7-O-glucuronide
Catalog No.:BCX0803
CAS No.:38934-20-2
- Coreopsin
Catalog No.:BCX0802
CAS No.:499-29-6
- Harmalan
Catalog No.:BCX0801
CAS No.:525-41-7
- Prosaikogenin G
Catalog No.:BCX0800
CAS No.:99365-23-8
- Pterin-6-carboxylic acid
Catalog No.:BCX0799
CAS No.:948-60-7
- 17-Hydroxygracillin
Catalog No.:BCX0798
CAS No.:90308-85-3
- Kanzonol D
Catalog No.:BCX0797
CAS No.:155233-20-8
- (-)-Epipodophyllotoxin
Catalog No.:BCX0796
CAS No.:4375-07-9
- Magnoloside B
Catalog No.:BCX0795
CAS No.:116872-05-0
- O-Cymen-5-ol
Catalog No.:BCX0794
CAS No.:39660-61-2
- 7,4′-Dihydroxyhomoisoflavane
Catalog No.:BCX0793
CAS No.:148462-00-4
- Neoeuonymine
Catalog No.:BCX0806
CAS No.:33510-25-7
- Thymoquinone
Catalog No.:BCX0807
CAS No.:490-91-5
- Monascin
Catalog No.:BCX0808
CAS No.:21516-68-7
- guan-fu base I
Catalog No.:BCX0809
CAS No.:110225-59-7
- Acetyl Dopamine Dimer I
Catalog No.:BCX0810
CAS No.:315188-82-0
- Monascorubrin
Catalog No.:BCX0811
CAS No.:13283-90-4
- 16-Hydroxyhexadecanoic acid
Catalog No.:BCX0812
CAS No.:506-13-8
- Xylotetraose
Catalog No.:BCX0813
CAS No.:22416-58-6
- Nepetalacton
Catalog No.:BCX0814
CAS No.:21651-62-7
- Kuwanon U
Catalog No.:BCX0815
CAS No.:123702-95-4
- Xylopentaose
Catalog No.:BCX0816
CAS No.:49694-20-4
- Hirudonucleodisulfide A
Catalog No.:BCX0817
CAS No.:1072789-37-7
Preparation of magnetic amphiphilic resin microspheres via the one-step polymerization method and extraction of four glucocorticoids for HPLC-MS analysis.[Pubmed:38458141]
J Chromatogr A. 2024 Apr 12;1720:464785.
Amphiphilic materials can be used for sample preparation of chromatography or mass spectrometry. Amphiphilic materials with magnetic properties in combination with magnetic suction devices allow for automated sample preparation. However, conventional synthesis methods are cumbersome and not suitable for the mass production of the material. In this study, a micro-suspension polymerization method was developed to synthesize magnetic amphiphilic resin microspheres (MARMs), providing new ideas for the preparation of amphiphilic microspheres. MARMs with particle sizes ranging from 3 to 6 mum were successfully prepared, with BET surface area up to 653.2 m(2)/g. A magnetic solid-phase extraction method based on MARM-5 was developed for the extraction of four glucocorticoids including Cortisone, Hydrocortisone, Cortodoxone, and Corticosterone. This method had a very short adsorption time of 0.5 min and a total extraction time of only 13 min. The limit of detection for the four glucocorticoids ranged from 0.22 to 0.82 ng/L. There was a good linear relationship between sample concentration and peak area in the range of 25 approximately 500 ng/L. Relative recovery of 98 % approximately 108 % and internal standard normalized matrix effect factors of 95 approximately 114 % were obtained, and the relative standard deviation was between 2.3 % and 6.3 %. The MARMs would be used as excellent solid extraction material for glucocorticoids.
Combined analysis of 16S rDNA sequencing and metabolomics to find biomarkers of drug-induced liver injury.[Pubmed:37704684]
Sci Rep. 2023 Sep 13;13(1):15138.
Drug induced liver injury (DILI) is a kind of liver dysfunction which caused by drugs, and gut microbiota could affect liver injury. However, the relationship between gut microbiota and its metabolites in DILI patients is not clear. The total gut microbiota DNA was extracted from 28 DILI patient and 28 healthy control volunteers (HC) and 16S rDNA gene were amplified. Next, differentially metabolites were screened. Finally, the correlations between the diagnostic strains and differentially metabolites were studied.The richness and uniformity of the bacterial communities decreased in DILI patients, and the structure of gut microbiota changed obviously. Enterococcus and Veillonella which belong to Firmicutes increased in DILI, and Blautia and Ralstonia which belong to Firmicutes, Dialister which belongs to Proteobacteria increased in HC. In addition, these diagnostic OTUs of DILI were associated with the DILI damage mechanism. On the other hands, there were 66 differentially metabolites between DILI and HC samples, and these metabolites were mainly enriched in pyrimidine metabolism and steroid hormone biosynthesis pathways. Furthermore, the collinear network map of the key microbiota-metabolites were constructed and the results indicated that Cortodoxone, Prostaglandin I1, Bioyclo Prostaglandin E2 and Anacardic acid were positively correlated with Blautia and Ralstonia, and negatively correlated with Veillonella.This study analyzed the changes of DILI from the perspective of gut microbiota and metabolites. Key strains and differentially metabolites of DILI were screened and the correlations between them were studied. This study further illustrated the mechanism of DILI.


