PurpurinCAS# 81-54-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 81-54-9 | SDF | Download SDF |
| PubChem ID | 6683 | Appearance | Red brown powder |
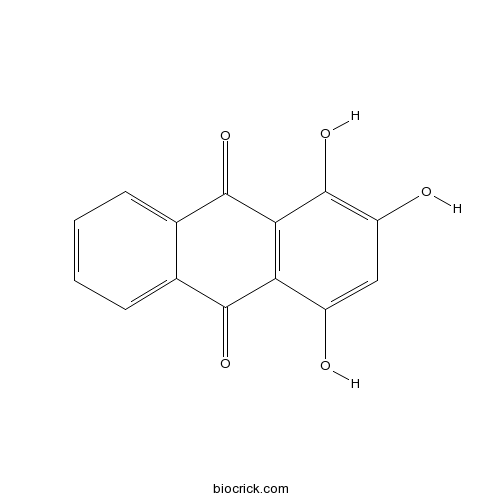
| Formula | C14H8O5 | M.Wt | 256.2 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Synonyms | Hydroxylizaric acid; 1,2,4-Trihydroxy 9,10-anthraquinone; Verantin | ||
| Solubility | DMSO : 125 mg/mL (487.88 mM; Need ultrasonic) | ||
| Chemical Name | 1,2,4-trihydroxyanthracene-9,10-dione | ||
| SMILES | C1=CC=C2C(=C1)C(=O)C3=C(C2=O)C(=C(C=C3O)O)O | ||
| Standard InChIKey | BBNQQADTFFCFGB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8O5/c15-8-5-9(16)14(19)11-10(8)12(17)6-3-1-2-4-7(6)13(11)18/h1-5,15-16,19H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Purpurin is one of the natural colorants extracted from madder roots and other Rubiaceae family plants. Purpurin is a novel specific inhibitor of Adipocyte-derived leucine aminopeptidase, it exhibits anti-angiogenic, antifungal, antibiotic, and antioxidative activities. |
| Targets | VEGFR | Antifection |
| In vitro | Effects of lovastatin, clomazone and methyl jasmonate treatment on the accumulation of purpurin and mollugin in cell suspension cultures of Rubia cordifolia.[Pubmed: 23845549]Chin J Nat Med. 2013 Jul;11(4):396-400.
Purpurin suppresses Candida albicans biofilm formation and hyphal development.[Pubmed: 23226409]PLoS One. 2012;7(11):e50866.We have previously demonstrated the novel antifungal activity of Purpurin against Candida fungi. |
| Kinase Assay | Purpurin inhibits adipocyte-derived leucine aminopeptidase and angiogenesis in a zebrafish model.[Pubmed: 24928393]Biochem Biophys Res Commun. 2014 Jul 18;450(1):561-7.A natural product Purpurin was identified as one of the most potent inhibitors of A-LAP from the screening. |
| Structure Identification | Spectrochim Acta A Mol Biomol Spectrosc. 2015 Apr 24.Structural and optical properties of Purpurin for dye-sensitized solar cells.[Pubmed: 26037779]
|

Purpurin Dilution Calculator

Purpurin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.9032 mL | 19.516 mL | 39.032 mL | 78.064 mL | 97.58 mL |
| 5 mM | 0.7806 mL | 3.9032 mL | 7.8064 mL | 15.6128 mL | 19.516 mL |
| 10 mM | 0.3903 mL | 1.9516 mL | 3.9032 mL | 7.8064 mL | 9.758 mL |
| 50 mM | 0.0781 mL | 0.3903 mL | 0.7806 mL | 1.5613 mL | 1.9516 mL |
| 100 mM | 0.039 mL | 0.1952 mL | 0.3903 mL | 0.7806 mL | 0.9758 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sennoside A
Catalog No.:BCN1002
CAS No.:81-27-6
- Cholic acid
Catalog No.:BCN1286
CAS No.:81-25-4
- Taurocholic acid
Catalog No.:BCN6954
CAS No.:81-24-3
- 2-Amino-1-naphthalenesulfonic acid
Catalog No.:BCC8519
CAS No.:81-16-3
- Musk ketone
Catalog No.:BCN8357
CAS No.:81-14-1
- 6-Amino-1-naphthalenesulfonic acid
Catalog No.:BCC8758
CAS No.:81-05-0
- 20R-Ginsenoside Rg2
Catalog No.:BCN2554
CAS No.:80952-72-3
- (20R)-Ginsenoside Rh1
Catalog No.:BCN3700
CAS No.:80952-71-2
- Zingibroside R1
Catalog No.:BCN3433
CAS No.:80930-74-1
- Gnetumontanin B
Catalog No.:BCN3756
CAS No.:809237-87-4
- Concanamycin A
Catalog No.:BCC3919
CAS No.:80890-47-7
- 3,22-Dihydroxyolean-12-en-29-oic acid
Catalog No.:BCN1347
CAS No.:808769-54-2
- Warfarin
Catalog No.:BCC5221
CAS No.:81-81-2
- Rhodamine B
Catalog No.:BCN7215
CAS No.:81-88-9
- 4-Hydroxy-4-(methoxycarbonylmethyl)cyclohexanone
Catalog No.:BCN1346
CAS No.:81053-14-7
- Methyl 4-prenyloxycinnamate
Catalog No.:BCN7520
CAS No.:81053-49-8
- EUK 134
Catalog No.:BCC4317
CAS No.:81065-76-1
- Kauniolide
Catalog No.:BCC5313
CAS No.:81066-45-7
- Pravastatin
Catalog No.:BCC4141
CAS No.:81093-37-0
- Cisapride
Catalog No.:BCC4207
CAS No.:81098-60-4
- Clarithromycin
Catalog No.:BCC9219
CAS No.:81103-11-9
- Racecadotril
Catalog No.:BCC4614
CAS No.:81110-73-8
- N-Nonyldeoxynojirimycin
Catalog No.:BCC7752
CAS No.:81117-35-3
- (Z)-Lachnophyllum lactone
Catalog No.:BCN4746
CAS No.:81122-95-4
Purpurin inhibits adipocyte-derived leucine aminopeptidase and angiogenesis in a zebrafish model.[Pubmed:24928393]
Biochem Biophys Res Commun. 2014 Jul 18;450(1):561-7.
Adipocyte-derived leucine aminopeptidase (A-LAP) is a novel member of the M1 family of zinc metallopeptidases, which has been reported to play a crucial role in angiogenesis. In the present study, we conducted a target-based screening of natural products and synthetic chemical libraries using the purified enzyme to search novel inhibitors of A-LAP. Amongst several hits isolated, a natural product Purpurin was identified as one of the most potent inhibitors of A-LAP from the screening. In vitro enzymatic analyses demonstrated that Purpurin inhibited A-LAP activity in a non-competitive manner with a Ki value of 20 M. In addition, Purpurin showed a strong selectivity toward A-LAP versus another member of M1 family of zinc metallopeptidase, aminopeptidase N (APN). In angiogenesis assays, Purpurin inhibited the vascular endothelial growth factor (VEGF)-induced invasion and tube formation of human umbilical vein endothelial cells (HUVEC). Moreover, Purpurin inhibited in vivo angiogenesis in zebrafish embryo without toxicity. These data demonstrate that Purpurin is a novel specific inhibitor of A-LAP and could be developed as a new anti-angiogenic agent.
Structural and optical properties of Purpurin for dye-sensitized solar cells.[Pubmed:26037779]
Spectrochim Acta A Mol Biomol Spectrosc. 2015 Oct 5;149:997-1008.
In this work, we reported a combined experimental and theoretical study on molecular structure, vibrational spectra and Homo-Lumo analysis of Purpurin and TiO2/Purpurin. The geometries, electronic structures, molecular orbital analysis of natural dye sensitizer Purpurin were studied based on density functional theory (DFT) using the hybrid functional B3LYP. Fourier transform infrared (FT-IR) and FT-Raman spectra have been recorded and extensive spectroscopic investigations have been carried out on Purpurin. The optimized geometries, wave number and intensity of the vibrational bands of Purpurin have been calculated using density functional level of theory (DFT/B3LYP) employing 6-311G (d,p) basis set. Based on the comparison between calculated and experimental results, assignments of the fundamental vibrational modes are examined. Features of the electronic absorption spectrum in the visible and near-UV regions were assigned based on TD-DFT calculations. The calculated results suggest that the three excited states with the lowest excited energies in 1,2,4, trihydroxy 9-10 anthraquinone was due to photo-induced electron transfer processes. Frontier molecular orbitals (FMO), LUMO, HOMO, and energy gap, of these dyes have been analyzed to show their effect on the process of electron injection and dye regeneration. Interaction between HOMO and LUMO of Purpurin are investigated to understand the recombination process and charge transfer process involving these dyes. We also performed analysis of I-V characteristics to investigate the role of charge transfer and the stability of the dye molecule.
Purpurin suppresses Candida albicans biofilm formation and hyphal development.[Pubmed:23226409]
PLoS One. 2012;7(11):e50866.
A striking and clinically relevant virulence trait of the human fungal pathogen Candida albicans is its ability to grow and switch reversibly among different morphological forms. Inhibition of yeast-to-hypha transition in C. albicans represents a new paradigm for antifungal intervention. We have previously demonstrated the novel antifungal activity of Purpurin against Candida fungi. In this study, we extended our investigation by examining the in vitro effect of Purpurin on C. albicans morphogenesis and biofilms. The susceptibility of C. albicans biofilms to Purpurin was examined quantitatively by 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide reduction assay. Hyphal formation and biofilm ultrastructure were examined qualitatively by scanning electron microscopy (SEM). Quantitative reverse transcription-PCR (qRT-PCR) was used to evaluate the expression of hypha-specific genes and hyphal regulator in Purpurin-treated fungal cells. The results showed that, at sub-lethal concentration (3 microg/ml), Purpurin blocked the yeast-to-hypha transition under hypha-inducing conditions. Purpurin also inhibited C. albicans biofilm formation and reduced the metabolic activity of mature biofilms in a concentration-dependent manner. SEM images showed that Purpurin-treated C. albicans biofilms were scanty and exclusively consisted of aggregates of blastospores. qRT-PCR analyses indicated that Purpurin downregulated the expression of hypha-specific genes (ALS3, ECE1, HWP1, HYR1) and the hyphal regulator RAS1. The data strongly suggested that Purpurin suppressed C. albicans morphogenesis and caused distorted biofilm formation. By virtue of the ability to block these two virulence traits in C. albicans, Purpurin may represent a potential candidate that deserves further investigations in the development of antifungal strategies against this notorious human fungal pathogen in vivo.
Effects of lovastatin, clomazone and methyl jasmonate treatment on the accumulation of purpurin and mollugin in cell suspension cultures of Rubia cordifolia.[Pubmed:23845549]
Chin J Nat Med. 2013 Jul;11(4):396-400.
AIM: To determine the IPP origin of the naphthoquinones (NQs) in Rubia cordifolia, and to evaluate the effects of methyl jasmonate (MeJA) treatment, MEP, and MVA pathway inhibitor treatment on the accumulation of anthraquinones (AQs) and NQs in cell suspension cultures of R. cordifolia. METHODS: Cell suspension cultures of R. cordifolia were established. Specific inhibitors (lovastatin and clomazone) and MeJA were supplied to the media, respectively. Treated cells were sampled every three days. Content determination of Purpurin (AQs) and mollugin (NQs) were carried out using RP-HPLC. The yield of the two compounds was compared with the DMSO-supplied group and the possible mechanism was discussed. RESULTS: Lovastatin treatment increased the yield of Purpurin and mollugin significantly. Clomazone treatment resulted in a remarkable decrease of both compounds. In the MeJA-treated cells, the Purpurin yield increased, meanwhile, the mollugin yield decreased compared with control. CONCLUSION: The IPP origin of mollugin in R. cordifolia cell suspension cultures was likely from the MEP pathway. To explain the different effects of MeJA on AQs and NQs accumulation, studies on the regulation and expression of the genes, especially after prenylation of 1,4-dihydroxy-2-naphthoic acid should be conducted.


