Senexin BCDK8/CDK19 inhibitor, highly potent, selective and orally available CAS# 1449228-40-3 |
- THZ1
Catalog No.:BCC4005
CAS No.:1604810-83-4
- SNS-032 (BMS-387032)
Catalog No.:BCC1152
CAS No.:345627-80-7
- AZD-5438
Catalog No.:BCC3689
CAS No.:602306-29-6
- Dinaciclib (SCH727965)
Catalog No.:BCC3765
CAS No.:779353-01-4
- PD 0332991 (Palbociclib) HCl
Catalog No.:BCC3680
CAS No.:827022-32-2
- Palbociclib (PD0332991) Isethionate
Catalog No.:BCC3698
CAS No.:827022-33-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1449228-40-3 | SDF | Download SDF |
| PubChem ID | 71661259 | Appearance | Powder |
| Formula | C27H26N6O | M.Wt | 450.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SNX2-1-165 | ||
| Solubility | >22.6mg/mL in DMSO | ||
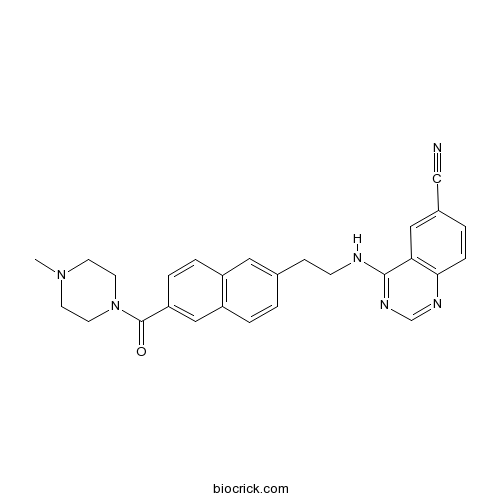
| Chemical Name | 4-[2-[6-(4-methylpiperazine-1-carbonyl)naphthalen-2-yl]ethylamino]quinazoline-6-carbonitrile | ||
| SMILES | CN1CCN(CC1)C(=O)C2=CC3=C(C=C2)C=C(C=C3)CCNC4=NC=NC5=C4C=C(C=C5)C#N | ||
| Standard InChIKey | VNADJTWHOAMTLY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H26N6O/c1-32-10-12-33(13-11-32)27(34)23-6-5-21-14-19(2-4-22(21)16-23)8-9-29-26-24-15-20(17-28)3-7-25(24)30-18-31-26/h2-7,14-16,18H,8-13H2,1H3,(H,29,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Senexin B Dilution Calculator

Senexin B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2196 mL | 11.098 mL | 22.1961 mL | 44.3922 mL | 55.4902 mL |
| 5 mM | 0.4439 mL | 2.2196 mL | 4.4392 mL | 8.8784 mL | 11.098 mL |
| 10 mM | 0.222 mL | 1.1098 mL | 2.2196 mL | 4.4392 mL | 5.549 mL |
| 50 mM | 0.0444 mL | 0.222 mL | 0.4439 mL | 0.8878 mL | 1.1098 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.222 mL | 0.4439 mL | 0.5549 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Senexin B is a highly potent, selective and orally available CDK8/CDK19 inhibitor with IC50 values ranging from 24-50 nM [1].
Cyclin-dependent kinase 8 (CDK8), along with its closely related isoform CDK19, is an oncogenic transcription-regulating kinase. CDK8 plays an essential role in the pluripotent stem cell phenotype. Also, CDK8 is a regulator of several transcriptional programs involved in carcinogenesis and has been identified as an oncogene in melanoma and colon cancer [1].
Senexin B is a highly potent, selective and orally available CDK8/CDK19 inhibitor. Senexin B exhibited selectivity for CDK8 and CDK19 with Kd values of 140 nM and 80 nM. Senexin B was fully soluble in 20% propylene glycol at 1 mM, and fully soluble in water at 50 mM concentration. Senexin B inhibited CDK8/19 in low nanomolar range in vitro and in vivo in an ATP competitive manner [2]. In HT1080 cells, Senexin B inhibited CMV-GFP expression induced by IPTG-inducible p21 [1].
In CB-17 SCID mice (8 weeks old), i.p. injections of Senexin B (40 mg/kg) or carrier for 5 days, mice were then injected s.c. with 1 x 106 cells of human A549 lung cancer cell line, Senexin B significantly slowed tumor growth. In female nude mice with the fat pad injected orthotopically with MDA-MB-468 triple-negative breast cancer (TNBC) cells, 5-day pretreatment with 25 mg/kg daily doses of Senexin B (i.p.) produced strong and sustained inhibition of tumor growth [1].
References:
1. Cdk8/cdk19 selective inhibitors and their use in anti-metastatic and chemopreventative methods for cancer. Publication number: WO2013116786 A1.
2. Donald C. Porter, Mengqian Chen, Jiaxin Liang, et al. Abstract PR08: Targeting tumor microenvironment with selective small-molecule inhibitors of CDK8/19.
- Androst-5-en-3-ol-7,17-dione acetate
Catalog No.:BCC8822
CAS No.:1449-61-2
- 24-Methylenecycloartan-3-ol
Catalog No.:BCN6254
CAS No.:1449-09-8
- 24-Methylenecycloartanone
Catalog No.:BCN7244
CAS No.:1449-08-7
- 21-Episerratenediol
Catalog No.:BCN6253
CAS No.:1449-06-5
- 18alpha-Glycyrrhetinic acid
Catalog No.:BCC8328
CAS No.:1449-05-4
- 1-Methyl-aminomethyl naphthalene
Catalog No.:BCC8471
CAS No.:14489-75-9
- ML314
Catalog No.:BCC4154
CAS No.:1448895-09-7
- MRS 2693 trisodium salt
Catalog No.:BCC7386
CAS No.:1448858-83-0
- 4',9,9'-Trihydroxy-3'-methoxy-3,7'-epoxy-4,8'-oxyneolignan
Catalog No.:BCN1563
CAS No.:144881-21-0
- Junipediol B
Catalog No.:BCN6252
CAS No.:144881-19-6
- Resiquimod (R-848)
Catalog No.:BCC4073
CAS No.:144875-48-9
- Garjasmin
Catalog No.:BCN6251
CAS No.:144868-43-9
- K145 hydrochloride
Catalog No.:BCC4072
CAS No.:1449240-68-9
- Chlojaponilactone B
Catalog No.:BCN7400
CAS No.:1449382-91-5
- Boeravinone O
Catalog No.:BCN6693
CAS No.:1449384-21-7
- DDR1-IN-1
Catalog No.:BCC5170
CAS No.:1449685-96-4
- DGAT1-IN-1
Catalog No.:BCC5511
CAS No.:1449779-49-0
- Pregnenolone
Catalog No.:BCN6255
CAS No.:145-13-1
- ω-Agatoxin IVA
Catalog No.:BCC7488
CAS No.:145017-83-0
- Digalactosyldiacylglycerol
Catalog No.:BCC8941
CAS No.:145033-48-3
- Fmoc-Asp(OMe)-OH
Catalog No.:BCC3090
CAS No.:145038-53-5
- FGIN-1-43
Catalog No.:BCC6739
CAS No.:145040-29-5
- Candesartan cilexetil
Catalog No.:BCC8900
CAS No.:145040-37-5
- CPI-169
Catalog No.:BCC5396
CAS No.:1450655-76-1
B-mode sonography wall thickness assessment of the temporal and axillary arteries for the diagnosis of giant cell arteritis: a cohort study.[Pubmed:28375835]
Clin Exp Rheumatol. 2017 Mar-Apr;35 Suppl 103(1):128-133. Epub 2017 Apr 4.
OBJECTIVES: We aimed to determine the diagnostic accuracy of B-mode compression sonography of the temporal arteries (tempCS) and B-mode sonographic measurement of the axillary artery intima media thickness (axIMT) for the diagnosis of giant cell arteritis (GCA). METHODS: After having established measurement of tempCS and axIMT in our routine diagnostic workup, 92 consecutive patients with a suspected diagnosis of GCA were investigated. Clinical characteristics were recorded and wall thickening of the temporal arteries (tempCS) and axillary arteries (axIMT) was measured (mm). Using the final clinical diagnosis as the reference standard, receiver operator characteristics (ROC) analysis was performed. In a subgroup of 26 patients interobserver agreement was assessed using Spearman's rank correlation. RESULTS: Cranial GCA, extracranial GCA, and combined cranial/extracranial GCA were diagnosed in 18, 7, and 9 individuals, respectively. For the diagnosis of cranial GCA, tempCS had an excellent area under the curve (AUC) of 0.95, with a cut-off of >/=0.7 mm offering a sensitivity and specificity of 85% and 95%. The AUC of axIMT for the diagnosis of extracranial GCA was 0.91 (cut-off >/=1.2 mm: sensitivity and specificity 81.3 and 96.1%). Applying a combined tempCS/axIMT cut-off of >/=0.7mm/1.2 mm, we calculated an overall sensitivity and specificity for the final clinical diagnosis of cranial and/or extracranial GCA of 85.3% and 91.4%. Interobserver agreement was strong for both parameters assessed (Spearman's rho 0.72 and 0.77, respectively). CONCLUSIONS: The combination of tempCS/axIMT allows objective sonographic assessment in suspected GCA with promising diagnostic accuracy.
Hepatitis B virus sequencing and liver fibrosis evaluation in HIV/HBV co-infected Nigerians.[Pubmed:28376292]
Trop Med Int Health. 2017 Jun;22(6):744-754.
OBJECTIVES: Molecular characteristics of hepatitis B virus (HBV), such as genotype and genomic mutations, may contribute to liver-related morbidity and mortality. The association of these characteristics with liver fibrosis severity in sub-Saharan Africa is uncertain. We aimed to characterise molecular HBV features in human immunodeficiency virus (HIV)/HBV co-infected Nigerians and evaluate associations between these characteristics and liver fibrosis severity before and after antiretroviral therapy (ART) initiation. METHODS: HIV/HBV co-infected Nigerians underwent liver fibrosis estimation by transient elastography (TE) prior to and 36 months after ART initiation. Basal core promoter/precore (BCP/PC) and preS1/preS2/S regions of HBV were sequenced from baseline plasma samples. We evaluated associations between HBV mutations and liver fibrosis severity by univariate and multivariable regression. RESULTS: At baseline, 94 patients underwent TE with median liver stiffness of 6.4 (IQR 4.7-8.7) kPa. Patients were predominantly infected with HBV genotype E (45/46) and HBe-antigen negative (75/94, 79.8%). We identified BCP A1762T/G1764A in 15/35 (43%), PC G1896A in 20/35 (57%), 'a' determinant mutations in 12/45 (26.7%) and preS2 deletions in 6/16 (37.5%). PreS2 mutations were associated with advanced fibrosis in multivariable analysis. At follow-up, median liver stiffness was 5.2 (IQR 4.1-6.6) kPa. No HBV molecular characteristics were associated with lack of fibrosis regression, although HIV virologic control, body mass index (BMI) and baseline CD4+ T-cell count were associated with a decline in fibrosis stage. CONCLUSION: Frequent BCP/PC and preS1/preS2/S mutations were found in ART-naive HIV/HBV co-infected Nigerians. Median liver stiffness declined after initiation of ART, regardless of pre-ART HBV mutational pattern or virologic characteristics.
Simultaneous determination of cucurbitacin B, E, I and E-glucoside in plant material and body fluids by HPLC-MS.[Pubmed:28376351]
J Chromatogr B Analyt Technol Biomed Life Sci. 2017 May 1;1052:128-134.
A selective and sensitive analytical method for the simultaneous determination of cucurbitacin B, E, I and E-glucoside in plant material and body fluids by HPLC-MS was developed. After liquid-liquid extraction with dichlormethane, separation was achieved on a Phenomenex Luna Pentafluorophenyl Column (150mmx2mm, 5mum) using acetonitrile-water (90:10, v/v) as mobile phase system. Detection was performed using a 3200 Q Trap mass spectrometer (AB Sciex). For analysis Q1 Scans with negative ionisation were chosen. The method was validated for serum as the matrix of choice. Limits of detection are in the picogram range, limits of quantification are between 0.05 and 0.42ng/mL, recoveries are above 50%. The assay was linear in the calibration range from 1.0 to 50ng/mL for cucurbitacin E and from 0.10 to 50ng/mL for the cucurbitacins B, I and E-glucoside. The applicability of the method was demonstrated by the determination of cucurbitacins in zucchini plant material and body fluids from intoxication cases.
The new frontier of epigenetic heterogeneity in B-cell neoplasms.[Pubmed:28375986]
Curr Opin Hematol. 2017 Jul;24(4):402-408.
PURPOSE OF REVIEW: There is mounting evidence that heterogeneity of the epigenome is a feature of many cancers, including B-cell lymphomas, and presents important clinical implications. The purpose of this review is to explain the biological and clinical relevance of this epigenetic phenomenon in B-cell neoplasms. RECENT FINDINGS: Here, we summarize new findings demonstrating that B-cell lymphomas display increased DNA methylation heterogeneity compared to their normal counterparts. This plasticity of cytosine methylation manifests both as intertumor and intratumor heterogeneity and is associated with worse prognosis and poor clinical outcome in lymphoma patients. Recent studies of different subtypes of B-cell lymphomas have revealed that epigenetic aberrations and heterogeneous cytosine methylation patterning are common features of all neoplasms derived from B-lymphocytes, irrespective of maturation stage. With regard to mechanisms driving this process, recent reports suggest that cytosine methylation heterogeneity arises through passive and active processes. One factor implicated in active generation of cytosine methylation heterogeneity is activation-induced cytidine deaminase, which mediates DNA methylation changes and introduces epigenetic heterogeneity in normal germinal center B cells, the cells of origin of mature B-cell neoplasms such as diffuse large B-cell lymphoma and follicular lymphoma. SUMMARY: Understanding the scope and mechanism of epigenetic heterogeneity in cancer is of paramount importance to our understanding of clonal plasticity and treatment responses in B-cell lymphomas.


