Sinapine thiocyanateCAS# 7431-77-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 7431-77-8 | SDF | Download SDF |
| PubChem ID | 73554029 | Appearance | Powder |
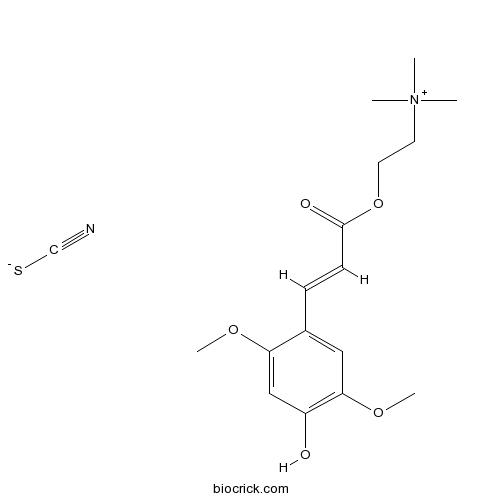
| Formula | C17H24N2O5S | M.Wt | 368.45 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 125 mg/mL (339.26 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 2-[(E)-3-(4-hydroxy-2,5-dimethoxyphenyl)prop-2-enoyl]oxyethyl-trimethylazanium;thiocyanate | ||
| SMILES | C[N+](C)(C)CCOC(=O)C=CC1=CC(=C(C=C1OC)O)OC.C(#N)[S-] | ||
| Standard InChIKey | NPOZDPAVPFLLCN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H23NO5.CHNS/c1-17(2,3)8-9-22-16(19)7-6-12-10-15(21-5)13(18)11-14(12)20-4;2-1-3/h6-7,10-11H,8-9H2,1-5H3;3H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sinapine thiocyanate has antioxidant activities and is hepatoprotective in a dose-dependent manner, implies that Brassica rapa seeds could be developed as a functional food for hepatoprotection. |
| In vitro | Stability of Sinapine Thiocyanate from Water Extract of Semen Raphani in Artificial Gastric and Intestinal Juice[Reference: WebLink]《Food and Drug》 2014-03To investigate the stability of Sinapine thiocyanate in artificial gastric and intestinal juice gained from the water extract of Semen Raphani. |
| Structure Identification | 《Strait Pharmaceutical Journal》 2013-03Determination of sinapine thiocyanate in YangHePingChuan granules by UPLC[Reference: WebLink]To establish a method for determination of Sinapine thiocyanate in YangHePingChuan granules by UPLC. |

Sinapine thiocyanate Dilution Calculator

Sinapine thiocyanate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7141 mL | 13.5704 mL | 27.1407 mL | 54.2814 mL | 67.8518 mL |
| 5 mM | 0.5428 mL | 2.7141 mL | 5.4281 mL | 10.8563 mL | 13.5704 mL |
| 10 mM | 0.2714 mL | 1.357 mL | 2.7141 mL | 5.4281 mL | 6.7852 mL |
| 50 mM | 0.0543 mL | 0.2714 mL | 0.5428 mL | 1.0856 mL | 1.357 mL |
| 100 mM | 0.0271 mL | 0.1357 mL | 0.2714 mL | 0.5428 mL | 0.6785 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sinapine is an alkaloid from seeds of the cruciferous species which shows favorable biological activities such as antioxidant and radio-protective activities.
In Vitro:Sinapine increases the sensitivity of Caco-2 cells to doxorubicin in a dose-dependent manner, whereas no or less effect is observed in the cells treated with doxorubicin alone. The combination of Sinapine and doxorubicin has a synergistic effect and increased the cytotoxicity of doxorubicin against Caco-2 cells. Results indicate that Sinapine plays an important role in the down-regulation of P-glycoprotein expression through suppression of FGFR4-FRS2a-ERK1/2 signaling pathway[1]. Sinapine can effectively protect against OH-induced damages to DNA and MSCs, thereby it may have a therapeutic potential in MSCs transplantation[2].
In Vivo:During the first 8 days, the dry matter intake and live weight gain of the rats are significantly reduced by the intake of sinapine and other phenolic compounds. However, after this adaptation period their performances are similar to those of the control group[3].
References:
[1]. Guo Y, et al. Sinapine as an active compound for inhibiting the proliferation of Caco-2 cells via downregulation of P-glycoprotein. Food Chem Toxicol. 2014 May;67:187-92.
[2]. Li X, et al. Protective Effect of Sinapine against Hydroxyl Radical-Induced Damage to Mesenchymal Stem Cells and Possible Mechanisms. Chem Pharm Bull (Tokyo). 2016;64(4):319-25.
[3]. Vermorel M, et al. Valorization of rapeseed meal. 5. Effects of sinapine and other phenolic compounds on food intake and nutrient utilization in growing rats. Reprod Nutr Dev. 1987;27(4):781-90.
- Triptophenolide
Catalog No.:BCN2546
CAS No.:74285-86-2
- 12-Oxograndiflorenic acid
Catalog No.:BCN7624
CAS No.:74284-42-7
- 7-Acetylintermedine
Catalog No.:BCN1998
CAS No.:74243-01-9
- p-Chlorophenylalanine
Catalog No.:BCC5689
CAS No.:7424-00-2
- Baogongteng A
Catalog No.:BCN1874
CAS No.:74239-84-2
- Doronenine
Catalog No.:BCN2066
CAS No.:74217-57-5
- OSU-03012 (AR-12)
Catalog No.:BCC1255
CAS No.:742112-33-0
- Carbenoxolone disodium
Catalog No.:BCC3745
CAS No.:7421-40-1
- Vintafolide
Catalog No.:BCC5265
CAS No.:742092-03-1
- 3-Bromo-7-nitroindazole
Catalog No.:BCC6770
CAS No.:74209-34-0
- Uplandicine
Catalog No.:BCN2055
CAS No.:74202-10-1
- Doxazosin
Catalog No.:BCC4218
CAS No.:74191-85-8
- Quercetin-3-gentiobioside
Catalog No.:BCN3878
CAS No.:7431-83-6
- Somatostatin 1-28
Catalog No.:BCC5715
CAS No.:74315-46-1
- Z-Trp-OH
Catalog No.:BCC2750
CAS No.:7432-21-5
- Schisandrin A
Catalog No.:BCN5815
CAS No.:7432-28-2
- 4',4'''-Di-O-methylcupressuflavone
Catalog No.:BCN4295
CAS No.:74336-91-7
- (RS)-AMPA
Catalog No.:BCC6560
CAS No.:74341-63-2
- Chidamide
Catalog No.:BCC6445
CAS No.:743420-02-2
- Ent-16Α,17-Dihydroxy-19-Kauranoic Acid
Catalog No.:BCC9227
CAS No.:74365-74-5
- 4-hydroxyephedrine hydrochloride
Catalog No.:BCC8103
CAS No.:7437-54-9
- 8-Geranyloxypsoralen
Catalog No.:BCN4296
CAS No.:7437-55-0
- Leuprolide Acetate
Catalog No.:BCC1701
CAS No.:74381-53-6
- PAF (C16)
Catalog No.:BCC7522
CAS No.:74389-68-7
Systematic screening and characterization of Qi-Li-Qiang-Xin capsule-related xenobiotics in rats by ultra-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry.[Pubmed:29787993]
J Chromatogr B Analyt Technol Biomed Life Sci. 2018 Jul 15;1090:56-64.
Qi-Li-Qiang-Xin capsule (QLQX), a well-known traditional Chinese medicine prescription (TCMP), is consisted of eleven commonly used herbal medicines, has been widely used for the treatment of chronic heart failure (CHF). However, the absorbed components and related metabolites after oral administration of QLQX are still remaining unknown. In the present work, a reliable and effective method using ultra performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry (UPLC/Q-TOF-MS) was established to identify QLQX-related xenobiotics in rats. Based on a representative structure based homologous xenobiotics identification (RSBHXI) strategy, a total of eleven compounds (salvianolic acid B, formononetin, benzoylmesaconine, alisol A, Sinapine thiocyanate, naringin, tanshinone IIA, ginsenoside Rg1, ginsenoside Rb1, astragaloside IV and periplocin), bearing different chemical core structures, were selected and investigated for their metabolism in vivo. And then, comprehensive metabolic profiles of the holistic multi-ingredients in QLQX were achieved. As a result, a total of 121 QLQX-related xenobiotics (47 prototypes and 74 metabolites) were identified or tentatively characterized, among them eight prototypes (mesaconine, hypaconine, songorine, fuziline, neoline, talatizamine formononetin, neocryptotanshinone) and two metabolites (calycosin-gluA, formononetin-guA) were relatively the main existing xenobiotics exposed in blood. All absorbed prototype constituents were mainly from six composed herbal medicines (Aconiti lateralis radix, Astragali radix, Ginseng radix, Alismatis rhizoma, Salvia miltiorrhiza radix, Periploca cortex). The main metabolic reactions were methylation, hydrogenation, hydroxylation, oxidization, sulfation and glucuronidation. This is the first study on in vivo metabolism of QLQX. These results enabled us to focus on several high exposure ingredients in the discovery of effective substances of QLQX, however further pharmacokinetic study on these QLQX-related xenobiotics are needed to be carried out.
Mechanism of the protective effects of the combined treatment with rhynchophylla total alkaloids and sinapine thiocyanate against a prothrombotic state caused by vascular endothelial cell inflammatory damage.[Pubmed:28587383]
Exp Ther Med. 2017 Jun;13(6):3081-3088.
The aim of the present study was to investigate the effect and the underlying mechanism of the combined treatment of rhynchophylla total alkaloids (RTA) and Sinapine thiocyanate for protection against a prothrombotic state (PTS) associated with the tumor necrosis factor-alpha (TNF-alpha)-induced inflammatory injury of vascular endothelial cells (VECs). A TNF-alpha-induced VEC inflammatory injury model was established, and cell morphology of VECs was evaluated using scanning electron microscopy. In addition, reverse transcription-quantitative polymerase chain reaction and western blot analysis were performed to examine the mRNA and protein expression of coagulation-related factors, including nuclear factor-kappaB (NF-kappaB), transforming growth factor-beta1 (TGF-beta1), tissue factor (TF), plasminogen activator inhibitor (PAI-1), protease-activation receptors (PAR-1) and protein kinase C (PKC-alpha) in VECs. Combined treatment with RTA and Sinapine thiocyanate was demonstrated to reduce, to a varying extent, the mRNA and protein expression of NF-kappaB, TGF-beta1, TF, PAR-1, PKC-alpha and PAI-1. Furthermore, combined treatment with RTA and Sinapine thiocyanate was able to downregulate the expression of coagulation-related factors in injured VECs, thereby inhibiting the PTS induced by vascular endothelial injury. The underlying mechanism is partially associated with the TF-mediated activation of the thrombin-receptor signaling pathway that suppresses coagulation during inflammation and balances fibrinolysis in order to inhibit fibrin generation and deposition.
[Chemical mechanisms involved in slow fire processing and pulverization of Brassica juncea].[Pubmed:25850264]
Zhongguo Zhong Yao Za Zhi. 2014 Nov;39(22):4345-8.
This article dealed with the effects of processing method and duration on the major bioactive components (sinigrin and Sinapine thiocyanate) in Brassica juncea. The contents of sinigrin and Sinapine thiocyanate in decoctions of raw and processed B. juncea were determined and compared by high performance liquid chromatography on a Alltima C18 column (4.6 mm x 250 mm, 5 microm) at 35 degrees C with the acetonitrile-0.1% phosphoric acid as the mobile phrase in gradient elution. The detection wavelength of sinigrin and Sinapine thiocyanate was set at 227 nm and 326 nm, and the flow rate was 1.0 mL x min(-1). It was found that with the extended processing duration, the contents of sinigrin and Sinapine thiocyanate first increased and then decreased: i.e., 0-2 minutes they increased gradually (for sinigrin, by 9.65% in processed products and 356. 10% in powder; for Sinapine thiocyanate, by 12.82% in processed products and 3.41% in powder), and achieved their highest content at 2 min; then, decreased during the next 5 minutes (for sinigrin, by 80.35% in processed products and 82.09% in powder; for Sinapine thiocyanate, by 14.29% in processed products and 17.54% in powder), suggesting that processing duration could significantly affect the contents of bioactive components in B. juncea, enzymatic hydrolysis of sinigrin when the seed is crushed in the present of moisture may be responsible for the content change. It is recommended that the slow fire should be the best processing method and the raw seed could be used directly in the water extracts related industrial production.
Skin penetration of topically applied white mustard extract and its effects on epidermal Langerhans cells and cytokines.[Pubmed:24076395]
Int J Pharm. 2013 Nov 30;457(1):136-42.
White mustard (Sinapis alba L.), a traditional Chinese medicine, is widely used in China for clinical prevention and treatment of the common winter diseases of asthma and bronchitis by percutaneous administration in the summer. The present study is to investigate the skin penetration behavior of white mustard extract to elucidate the possible mechanism underlying its immune regulation activity. The principle active compound of the extract, Sinapine thiocyanate (ST), was used as a marker. The skin penetration of ST in white mustard extract was examined in vitro and in vivo. In vitro study on excised guinea pig hairless skin using Franz diffusion cell revealed ST can permeate through the skin and also accumulate in the skin. In vivo study was carried out on the guinea pig hairless skin for 24 h, and then skin was excised for frozen section, ST from the sections were extracted to quantify the amount of drug in different skin layers. The detailed distribution of ST showed that it accumulated in the epidermis, especially in the stratum corneum. After treatment with white mustard extract for 24h, the skin was stained with ATPase, and the morphometric parameters of epidermal LCs were compared to the untreated control through image-analysis system. A statistically significant reduction in LC density and increase in shape factor were observed. Cytokines related to LCs migration including interleukin 1beta (IL-1beta) and tumor necrosis factor alpha (TNF-alpha) were also measured after white mustard extract treated at different time points. Compared to the untreated group, white mustard extract significantly enhanced the release of IL-1beta and TNFalpha. The morphometric changes of LCs and the local cytokine release after topical white mustard treatment may explain the activity of the white mustard extract against asthma and bronchitis.


