Baogongteng ACAS# 74239-84-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 74239-84-2 | SDF | Download SDF |
| PubChem ID | 156282 | Appearance | Powder |
| Formula | C9H15NO3 | M.Wt | 185.22 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
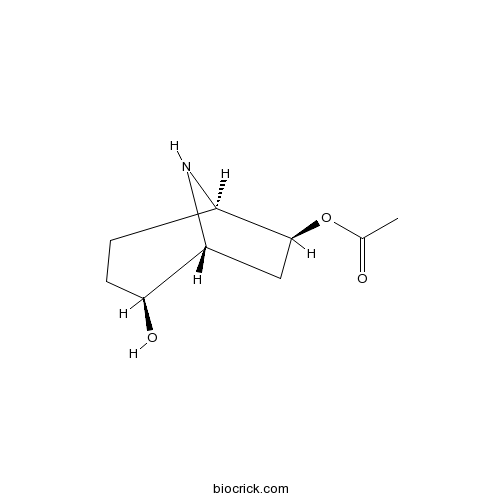
| Chemical Name | [(1R,4S,5R,7S)-4-hydroxy-8-azabicyclo[3.2.1]octan-7-yl] acetate | ||
| SMILES | CC(=O)OC1CC2C(CCC1N2)O | ||
| Standard InChIKey | FSXBMHMVOFJROW-HXFLIBJXSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Baogongteng A is a new tropane alkaloid used for treating glaucoma, synthetic baogongteng A also shows myotic activities in rabbits, but the potency is half of that of the natural product. 2. Baogongteng A and Baogongteng C are the major toxic chemical compounds of the Erycibe species. 3. Baogongteng A analogs as effective muscarinic agonists or antagonists in clinical use. |
| Targets | AChR |

Baogongteng A Dilution Calculator

Baogongteng A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.399 mL | 26.9949 mL | 53.9898 mL | 107.9797 mL | 134.9746 mL |
| 5 mM | 1.0798 mL | 5.399 mL | 10.798 mL | 21.5959 mL | 26.9949 mL |
| 10 mM | 0.5399 mL | 2.6995 mL | 5.399 mL | 10.798 mL | 13.4975 mL |
| 50 mM | 0.108 mL | 0.5399 mL | 1.0798 mL | 2.1596 mL | 2.6995 mL |
| 100 mM | 0.054 mL | 0.2699 mL | 0.5399 mL | 1.0798 mL | 1.3497 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Doronenine
Catalog No.:BCN2066
CAS No.:74217-57-5
- OSU-03012 (AR-12)
Catalog No.:BCC1255
CAS No.:742112-33-0
- Carbenoxolone disodium
Catalog No.:BCC3745
CAS No.:7421-40-1
- Vintafolide
Catalog No.:BCC5265
CAS No.:742092-03-1
- 3-Bromo-7-nitroindazole
Catalog No.:BCC6770
CAS No.:74209-34-0
- Uplandicine
Catalog No.:BCN2055
CAS No.:74202-10-1
- Doxazosin
Catalog No.:BCC4218
CAS No.:74191-85-8
- Cyclokievitone
Catalog No.:BCC8159
CAS No.:74175-82-9
- Haginin A
Catalog No.:BCN6861
CAS No.:74174-29-1
- R547
Catalog No.:BCC3927
CAS No.:741713-40-6
- 2,3-Dehydrokievitone
Catalog No.:BCN4294
CAS No.:74161-25-4
- Pimobendan
Catalog No.:BCC2294
CAS No.:74150-27-9
- p-Chlorophenylalanine
Catalog No.:BCC5689
CAS No.:7424-00-2
- 7-Acetylintermedine
Catalog No.:BCN1998
CAS No.:74243-01-9
- 12-Oxograndiflorenic acid
Catalog No.:BCN7624
CAS No.:74284-42-7
- Triptophenolide
Catalog No.:BCN2546
CAS No.:74285-86-2
- Sinapine thiocyanate
Catalog No.:BCN2765
CAS No.:7431-77-8
- Quercetin-3-gentiobioside
Catalog No.:BCN3878
CAS No.:7431-83-6
- Somatostatin 1-28
Catalog No.:BCC5715
CAS No.:74315-46-1
- Z-Trp-OH
Catalog No.:BCC2750
CAS No.:7432-21-5
- Schisandrin A
Catalog No.:BCN5815
CAS No.:7432-28-2
- 4',4'''-Di-O-methylcupressuflavone
Catalog No.:BCN4295
CAS No.:74336-91-7
- (RS)-AMPA
Catalog No.:BCC6560
CAS No.:74341-63-2
- Chidamide
Catalog No.:BCC6445
CAS No.:743420-02-2
[The preparation and bioactivities of chiral analogs of baogongteng A].[Pubmed:12016943]
Yao Xue Xue Bao. 1998 Nov;33(11):832-5.
Eight chiral analogs of Baogongteng A were prepared from (+/-)-3 alpha-hydroxy-6 beta-acetoxytropane by chemical resolution. In myotic or mydriatic tests in rabbits, (-)-3 alpha-paramethyl benzenesulfonyloxy-6 beta-acetoxytropane showed cholinergic activities, while (+)-3 alpha-benzoyloxy-6 beta-acetoxytropane and (+)-3 alpha-parachloro benzoyloxy-6 beta-acetoxytropane showed anticholinergic activities.
The absolute configuration plays an important role in muscarinic activity of BGT-A and its analogs.[Pubmed:19013075]
Bioorg Med Chem. 2008 Dec 15;16(24):10251-6.
Both enantiomers of 2, 3, and 4, three bioactive analogs of muscarinic agonist BGT-A were prepared respectively and underwent functional studies and radioreceptor binding assays. 6S enantiomers of 2, 3, and 4 showed obvious muscarinic activity, while 6R ones elicited little muscarinic activity by functional studies. Besides, the affinity of 6S enantiomers of 2, 3, and 4 was greatly larger than that of their 6R enantiomers respectively. All these pharmacological results indicated the 6S configuration was beneficial for the active BGT-A analogs to bind with the muscarinic receptors. The finding was in good agreement with our previous SAR study to BGT-A and its active analogs by computational approach. The understanding to the relationship between muscarinic activity and absolute configuration will provide the basis for successive screening of BGT-A analogs as effective muscarinic agonists or antagonists in clinical use.
[Studies on synthesis of baogongteng A--a new myotic agent].[Pubmed:2801130]
Yao Xue Xue Bao. 1989;24(2):105-9.
Baogongteng A, isolated from Erycibe obtusifolia Benth., is a new tropane alkaloid used for treating glaucoma. Using synthetic 6 beta-acetoxy-tropinone as starting material, racemic Baogongteng A(8) was synthesized. Synthetic Baogongteng A also shows myotic activities in rabbits, but the potency is half of that of the natural product.
Toxicology and the chemical foundation of plants of Erycibe.[Pubmed:25073109]
Regul Toxicol Pharmacol. 2014 Oct;70(1):349-56.
Erycibe is a relatively small genus in the family Convolvulaceae with over 10 identified species. Some Erycibe plant species are purportedly toxic at high doses. However, few toxicology studies have been conducted on those species. In this study, the toxicity of 40% ethanolic extracts of Erycibeobtusifolia, Erycibeschmidtii, and Erycibeellipptimba was evaluated. E. ellipptimba has been reported to be more toxic due to containing larger amounts of Baogongteng C, an alkaloid with known toxicity. Thus, E. ellipptimba was chosen for further toxicology study here. An HPLC-MS method was developed to identify the main components and determine the percentages of Baogongteng C in total alkaloid of E. ellipptimba (EWA). The toxicity of total alkaloid and Baogongteng C was evaluated and compared. The results indicated that Baogongteng A and Baogongteng C are the major toxic chemical compounds of the Erycibe species tested. The results also suggest EWA is cholinergic. Finally, in a subacute toxicity study of EWA, alterations observed with high dosage suggest that the liver and kidney could be the target organs of toxicity.


