StachybotrylactamCAS# 163391-76-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 163391-76-2 | SDF | Download SDF |
| PubChem ID | 45934402 | Appearance | Powder |
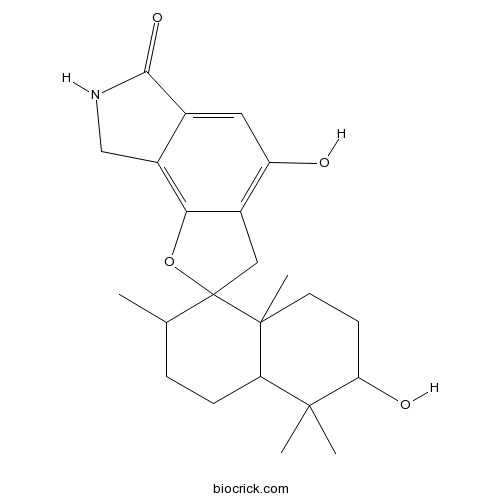
| Formula | C23H31NO4 | M.Wt | 385.50 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 3,4'-dihydroxy-4,4,7,8a-tetramethylspiro[2,3,4a,5,6,7-hexahydro-1H-naphthalene-8,2'-7,8-dihydro-3H-furo[2,3-e]isoindole]-6'-one | ||
| SMILES | CC1CCC2C(C(CCC2(C13CC4=C(C=C5C(=C4O3)CNC5=O)O)C)O)(C)C | ||
| Standard InChIKey | ZSVLMDBFFSSVAK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H31NO4/c1-12-5-6-17-21(2,3)18(26)7-8-22(17,4)23(12)10-14-16(25)9-13-15(19(14)28-23)11-24-20(13)27/h9,12,17-18,25-26H,5-8,10-11H2,1-4H3,(H,24,27) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Stachybotrylactam has toxicity. |

Stachybotrylactam Dilution Calculator

Stachybotrylactam Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.594 mL | 12.9702 mL | 25.9403 mL | 51.8807 mL | 64.8508 mL |
| 5 mM | 0.5188 mL | 2.594 mL | 5.1881 mL | 10.3761 mL | 12.9702 mL |
| 10 mM | 0.2594 mL | 1.297 mL | 2.594 mL | 5.1881 mL | 6.4851 mL |
| 50 mM | 0.0519 mL | 0.2594 mL | 0.5188 mL | 1.0376 mL | 1.297 mL |
| 100 mM | 0.0259 mL | 0.1297 mL | 0.2594 mL | 0.5188 mL | 0.6485 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- FIIN-2
Catalog No.:BCC3974
CAS No.:1633044-56-0
- Bethoxazin
Catalog No.:BCC5471
CAS No.:163269-30-5
- Sitafloxacin Hydrate
Catalog No.:BCC4959
CAS No.:163253-35-8
- Clevudine
Catalog No.:BCC4770
CAS No.:163252-36-6
- 680C91
Catalog No.:BCC6158
CAS No.:163239-22-3
- Ezetimibe
Catalog No.:BCN2180
CAS No.:163222-33-1
- Cimifugin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN7853
CAS No.:1632110-81-6
- (-)-[3R,4S]-Chromanol 293B
Catalog No.:BCC7080
CAS No.:163163-24-4
- Chromanol 293B
Catalog No.:BCC7055
CAS No.:163163-23-3
- Cannabisin F
Catalog No.:BCN4696
CAS No.:163136-19-4
- BYK 49187
Catalog No.:BCC2450
CAS No.:163120-31-8
- N-Benzylmaleimide
Catalog No.:BCC9095
CAS No.:1631-26-1
- 2',4'-Di-O-(E-p-coumaroyl)afzelin
Catalog No.:BCN6512
CAS No.:163434-73-9
- Fmoc-Met(O2)-OH
Catalog No.:BCC3531
CAS No.:163437-14-7
- Flufenamic acid
Catalog No.:BCC9162
CAS No.:530-78-9
- Triptoquinonide
Catalog No.:BCN1724
CAS No.:163513-81-3
- Vilazodone Hydrochloride
Catalog No.:BCC2041
CAS No.:163521-08-2
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
- Fmoc-D-Trp(Boc)-OH
Catalog No.:BCC3561
CAS No.:163619-04-3
- Evofolin C
Catalog No.:BCN4695
CAS No.:163634-05-7
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
- Pazufloxacin mesilate
Catalog No.:BCC9114
CAS No.:163680-77-1
- WIN 64338 hydrochloride
Catalog No.:BCC6914
CAS No.:163727-74-0
Spirocyclic drimanes from the marine fungus Stachybotrys sp. strain MF347.[Pubmed:24694571]
Mar Drugs. 2014 Apr 1;12(4):1924-38.
A novel spirocyclic drimane coupled by two drimane fragment building blocks 2 and a new drimane 1 were identified in mycelia and culture broth of Stachybotrys sp. MF347. Their structures were established by spectroscopic means. This is the first example of spirocyclic drimane coupled by a spirodihydrobenzofuranlactam unit and a spirodihydroisobenzofuran unit; and the connecting position being N-C instead of an N and N connecting unit. Strain MF347 produced also the known spirocyclic drimanes stachybocin A (12) and stachybocin B (11) featured by two sesquiterpene-spirobenzofuran structural units connected by a lysine residue; the known spirocyclic drimanes chartarlactam O (5); chartarlactam K (6); F1839A (7); Stachybotrylactam (8); stachybotramide (9); and 2alpha-acetoxyStachybotrylactam acetate (10); as well as ilicicolin B (13), a known sesquiterpene. The relative configuration of two known spirobenzofuranlactams (3 and 4) was determined. All compounds were subjected to biological activity tests. The spirocyclic drimane 2, 11, and 12, as well as the sesquiterpene 13, exhibited antibacterial activity against the clinically relevant methicillin-resistant Staphylococcus aureus (MRSA).
Evaluation of settled floor dust for the presence of microbial metabolites and volatile anthropogenic chemicals in indoor environments by LC-MS/MS and GC-MS methods.[Pubmed:21872054]
Talanta. 2011 Sep 30;85(4):2027-38.
This study reports on detection of a large number of biological and anthropogenic pollutants using LC-MS/MS and GC-MS technologies in settled floor dust (SFD). The latter technique was applied to obtain a general picture on the presence of microbial as well as non-microbial volatile organic compounds, whereas the targeted LC-MS/MS analysis focused on identification of species specific secondary metabolites. In the absence of moisture monitoring data the relevance of finding of Stachybotrylactam and other metabolites of tertiary colonizers are confined only to accidental direct exposure to SFD. To the best of our knowledge 30 of the 71 identified volatile organic compounds (VOCs) are newly reported in SFD matrix. Coordinated application of "AMDIS and Spectconnect" was found beneficial for the evaluation and identification of prime volatile pollutants in complex environmental samples. Principal component analysis (PCA) of peak areas of 18 microbial volatile organic compounds (MVOCs) resulted in identification of nonanal as potential MVOC marker. Two more volatiles toluene and 1-tetradecanol though had discriminative influence, are not regarded as MVOC markers, considering their probable alternate origin from paints and cosmetics, respectively.
Total synthesis and structure revision of stachybotrys spirolactams.[Pubmed:12968895]
J Org Chem. 2003 Sep 19;68(19):7422-7.
The spirolactam structure 1 reported in 1996 by Roggo et al. has been enantioselectively synthesized in 20 steps from (+)-Wieland-Miescher ketone. Spectra of this synthetic lactam differed from those of the natural spirolactam from the Roggo group, leading to the hypothesis that the natural lactam may be the regioisomer 27, a structure previously ascribed by Jarvis et al. to the natural product Stachybotrylactam. This hypothesis was confirmed by the first total synthesis of (-)-Stachybotrylactam (27). Double reductive amination of two molecules of aldehyde 29 with one molecule of l-lysine led by analogous chemistry to the first synthesis of the pseudosymmetric "dimer" 30, whose spectra corresponded to those of the natural "dimer" originally assigned structure 5 by the Roggo group.


