BethoxazinCAS# 163269-30-5 |
- Amyloid β-Peptide (10-20) (human)
Catalog No.:BCC1026
CAS No.:152286-31-2
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
- Myelin Basic Protein (68-82), guinea pig
Catalog No.:BCC1020
CAS No.:98474-59-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 163269-30-5 | SDF | Download SDF |
| PubChem ID | 12040175 | Appearance | Powder |
| Formula | C11H9NO2S2 | M.Wt | 251.32 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
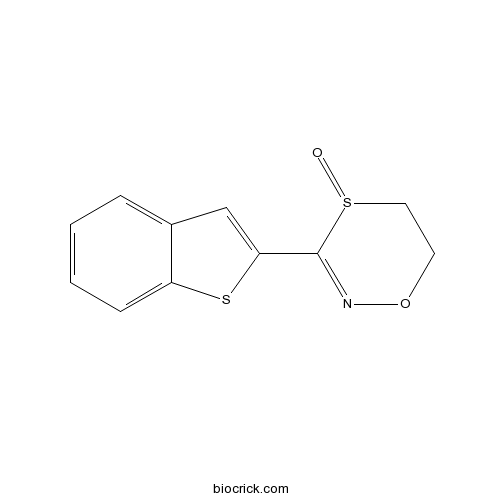
| Chemical Name | 3-(1-benzothiophen-2-yl)-5,6-dihydro-1,4,2-oxathiazine 4-oxide | ||
| SMILES | C1CS(=O)C(=NO1)C2=CC3=CC=CC=C3S2 | ||
| Standard InChIKey | NRAYWXLNSHEHQO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H9NO2S2/c13-16-6-5-14-12-11(16)10-7-8-3-1-2-4-9(8)15-10/h1-4,7H,5-6H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Bethoxazin Dilution Calculator

Bethoxazin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.979 mL | 19.895 mL | 39.7899 mL | 79.5798 mL | 99.4748 mL |
| 5 mM | 0.7958 mL | 3.979 mL | 7.958 mL | 15.916 mL | 19.895 mL |
| 10 mM | 0.3979 mL | 1.9895 mL | 3.979 mL | 7.958 mL | 9.9475 mL |
| 50 mM | 0.0796 mL | 0.3979 mL | 0.7958 mL | 1.5916 mL | 1.9895 mL |
| 100 mM | 0.0398 mL | 0.1989 mL | 0.3979 mL | 0.7958 mL | 0.9947 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Bethoxazin(Bethoguard) is a new broad spectrum industrial microbicide with applications in material and coating preservation.
- Sitafloxacin Hydrate
Catalog No.:BCC4959
CAS No.:163253-35-8
- Clevudine
Catalog No.:BCC4770
CAS No.:163252-36-6
- 680C91
Catalog No.:BCC6158
CAS No.:163239-22-3
- Ezetimibe
Catalog No.:BCN2180
CAS No.:163222-33-1
- Cimifugin 4'-O-beta-D-glucopyranoside
Catalog No.:BCN7853
CAS No.:1632110-81-6
- (-)-[3R,4S]-Chromanol 293B
Catalog No.:BCC7080
CAS No.:163163-24-4
- Chromanol 293B
Catalog No.:BCC7055
CAS No.:163163-23-3
- Cannabisin F
Catalog No.:BCN4696
CAS No.:163136-19-4
- BYK 49187
Catalog No.:BCC2450
CAS No.:163120-31-8
- N-Benzylmaleimide
Catalog No.:BCC9095
CAS No.:1631-26-1
- Albatrelin G
Catalog No.:BCN7596
CAS No.:1630970-05-6
- 17-Hydroxy-18-dehydroneogrifolin
Catalog No.:BCN7633
CAS No.:1630936-42-3
- FIIN-2
Catalog No.:BCC3974
CAS No.:1633044-56-0
- Stachybotrylactam
Catalog No.:BCN6967
CAS No.:163391-76-2
- 2',4'-Di-O-(E-p-coumaroyl)afzelin
Catalog No.:BCN6512
CAS No.:163434-73-9
- Fmoc-Met(O2)-OH
Catalog No.:BCC3531
CAS No.:163437-14-7
- Flufenamic acid
Catalog No.:BCC9162
CAS No.:530-78-9
- Triptoquinonide
Catalog No.:BCN1724
CAS No.:163513-81-3
- Vilazodone Hydrochloride
Catalog No.:BCC2041
CAS No.:163521-08-2
- Vilazodone
Catalog No.:BCC2040
CAS No.:163521-12-8
- Kadsulignan N
Catalog No.:BCN3631
CAS No.:163564-58-7
- Fmoc-D-Trp(Boc)-OH
Catalog No.:BCC3561
CAS No.:163619-04-3
- Evofolin C
Catalog No.:BCN4695
CAS No.:163634-05-7
- Kadsulignan L
Catalog No.:BCN3627
CAS No.:163660-06-8
Chemical reactivity and microbicidal action of bethoxazin.[Pubmed:22264763]
Bioorg Med Chem. 2012 Feb 15;20(4):1494-501.
Bethoxazin is a new broad spectrum industrial microbicide with applications in material and coating preservation. However, little is known of its reactivity profile and mechanism of action. In this study, we examined the reactivity of Bethoxazin toward biologically important nucleophilic groups using UV-vis spectroscopy and LC-MS/MS techniques and found the molecule to be highly electrophilic. Bethoxazin reacted with molecules containing free sulfhydryl groups such as GSH and human serum albumin to form covalent adducts that were detectable by MS, but did not react with amino, carboxylic, phenolic, amino oxo, alcoholic, and phosphate functional groups. Bethoxazin potently inhibited the catalytic activity of yeast DNA topoisomerase II and the growth of yeast BY4742 cells at low micromolar concentrations. However, the reduced form of Bethoxazin and GSH-treated Bethoxazin were both inactive in these assays. The experimentally determined relative reactivity of Bethoxazin and its reduced form analog correlated with their biological activities as well as their quantum-mechanically calculated electrophilicity properties. Taken together, the results suggest that Bethoxazin may exert its microbicidal action by reacting with sensitive endogenous sulfhydryl biomolecules of microbial cells. Consistent with this view, the inhibitory activity of Bethoxazin on topoisomerase II may be due to its ability to react with critical free cysteine sulfhydryl groups on the enzyme. Our studies have provided for the first time a better understanding of the reactivity of Bethoxazin, as well as some insights into the mechanism by which the compound exerts its microbicidal action.


