Euonymus alatus
Euonymus alatus
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
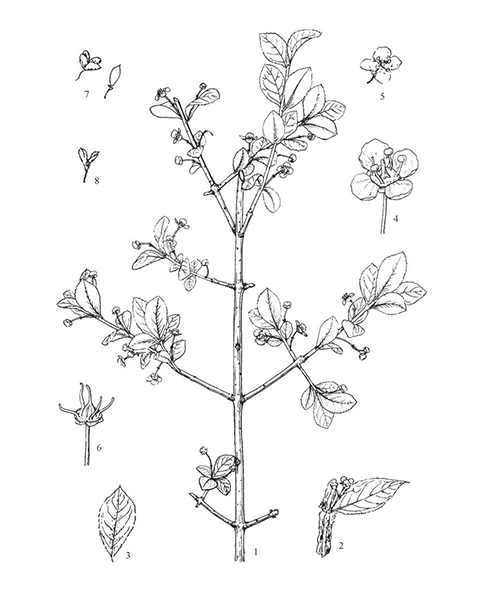
Natural products/compounds from Euonymus alatus
- Cat.No. Product Name CAS Number COA
-
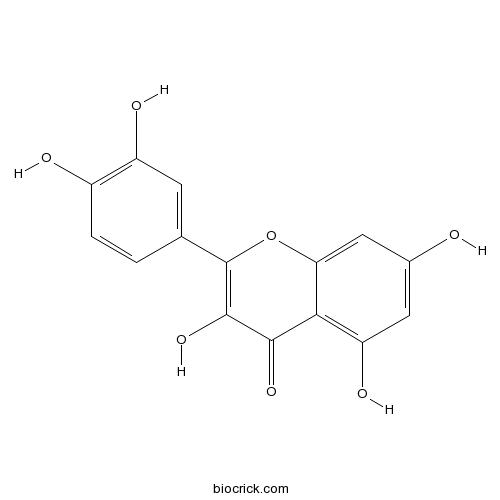
BCN6049
Quercetin117-39-5
Instructions
-
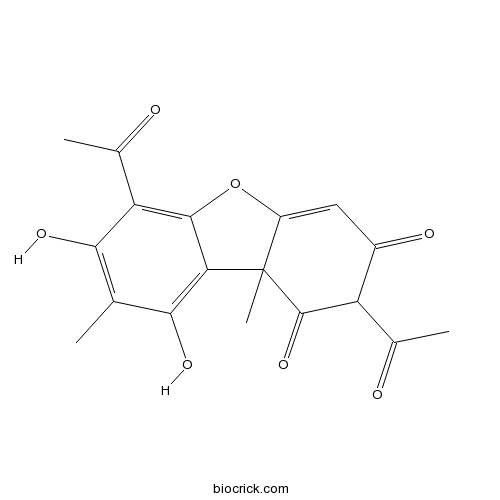
BCC8264
Usnic acid125-46-2
Instructions
-
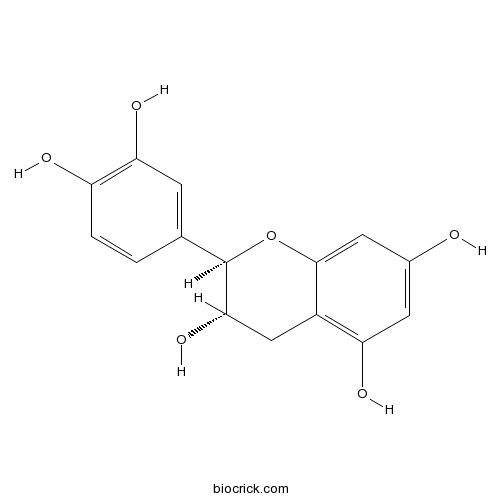
BCN1688
Catechin154-23-4
Instructions
-
BCN1717
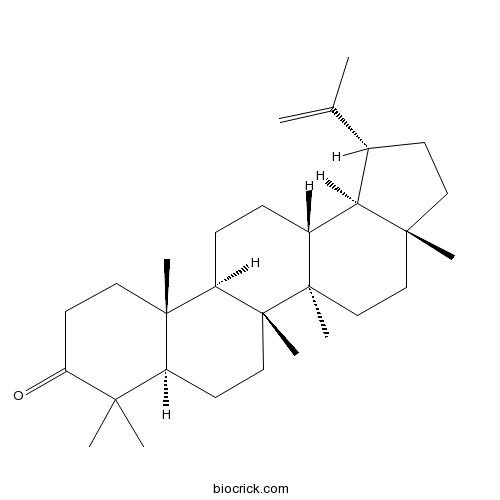
Lupenone1617-70-5
Instructions
-
BCN2298
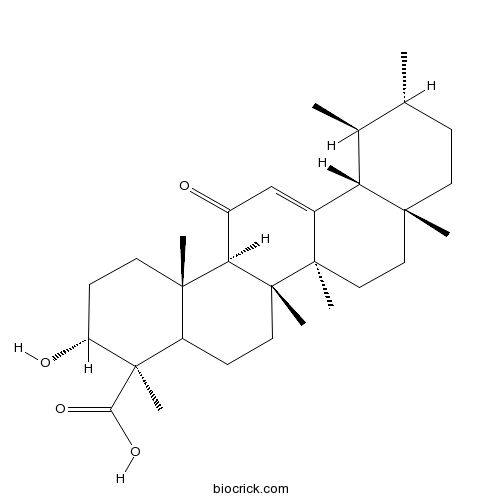
11-Keto-beta-boswellic acid17019-92-0
Instructions
-
BCN3087
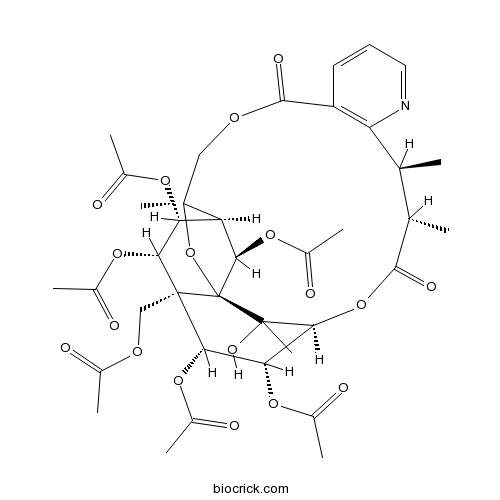
Evonine33458-82-1
Instructions
-
BCN5958
Puerarin3681-99-0
Instructions

-
BCN3083
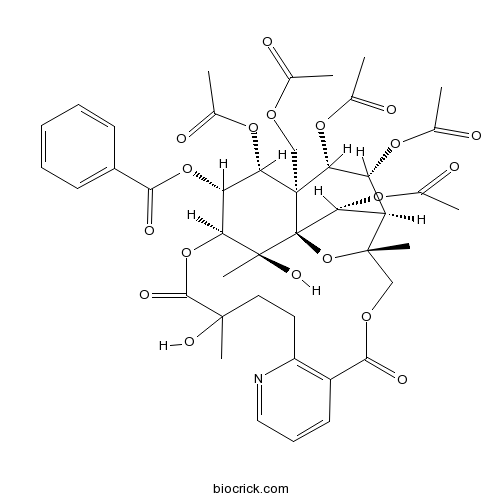
Wilfordine37239-51-3
Instructions
-
BCN5524
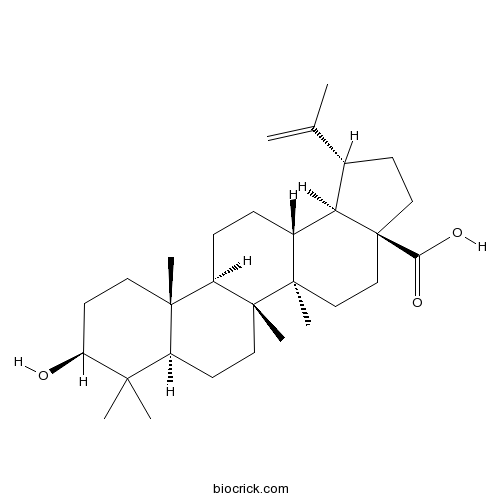
Betulinic acid472-15-1
Instructions
-
BCN5552
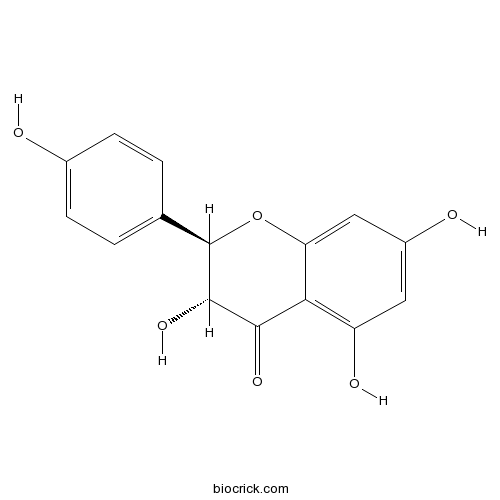
Aromadendrin480-20-6
Instructions
-
BCN5554
Linarin480-36-4
Instructions

-
BCN5564
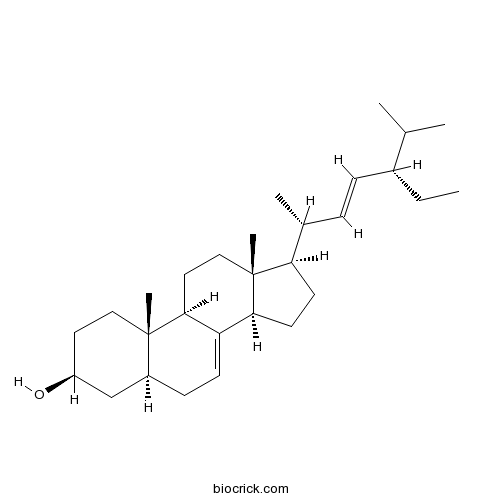
alpha-Spinasterol481-18-5
Instructions
-
BCN5570
Hyperoside482-36-0
Instructions

-
BCN5616
Oleanolic acid508-02-1
Instructions

-
BCN5636
Taraxerone514-07-8
Instructions

-
BCN5653
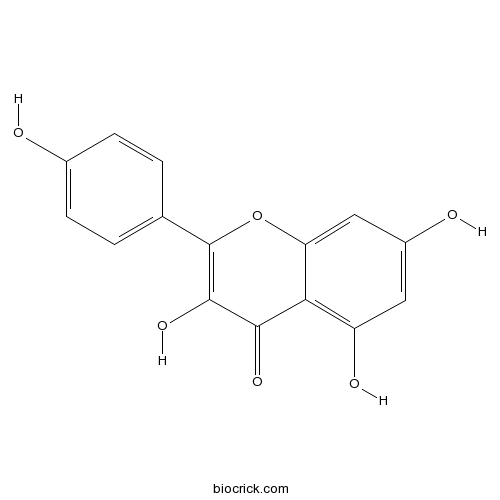
Kaempferol520-18-3
Instructions
-
BCN5665
Quercitrin522-12-3
Instructions
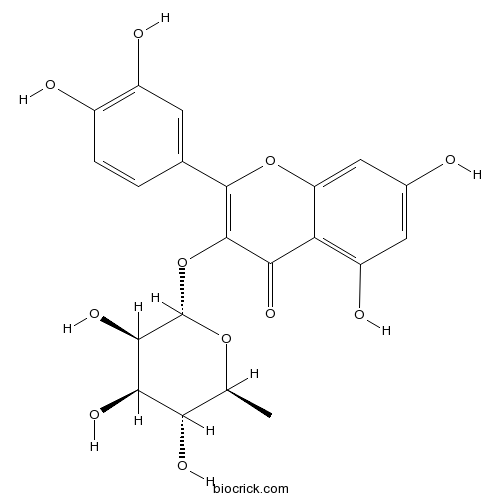
-
BCN5725
Lupeol545-47-1
Instructions

-
BCN5747
Friedelin559-74-0
Instructions

-
BCN5807
Caffeine58-08-2
Instructions

-
BCN1381
3-O-Acetyl-11-keto-beta-boswellic acid67416-61-9
Instructions

-
BCN4383
Wilforlide A84104-71-2
Instructions
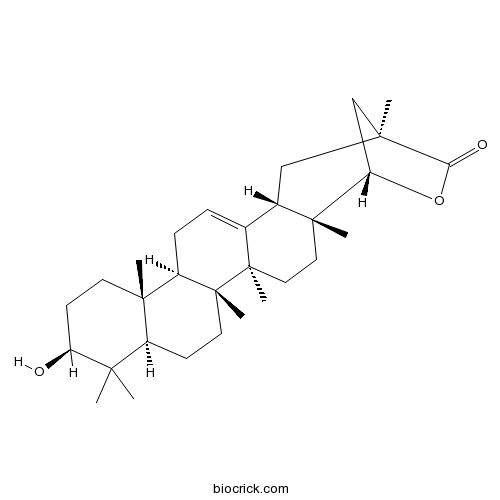
-
BCN4546
4-Hydroxybenzoic acid99-96-7
Instructions

Towards the synthetic design of camelina oil enriched in tailored acetyl-triacylglycerols with medium-chain fatty acids.[Pubmed: 29982623]
The ability to manipulate expression of key biosynthetic enzymes has allowed the development of genetically modified plants that synthesise unusual lipids that are useful for biofuel and industrial applications. By taking advantage of the unique activities of enzymes from different species, tailored lipids with a targeted structure can be conceived. In this study we demonstrate the successful implementation of such an approach by metabolically engineering the oilseed crop Camelina sativa to produce 3-acetyl-1,2-diacyl-sn-glycerols (acetyl-TAGs) with medium-chain fatty acids (MCFAs). Different transgenic camelina lines that had been genetically modified to produce MCFAs through the expression of MCFA-specific thioesterases and acyltransferases were retransformed with the Euonymus alatus gene for diacylglycerol acetyltransferase (EaDAcT) that synthesises acetyl-TAGs. Concomitant RNAi suppression of acyl-CoA:diacylglycerol acyltransferase increased the levels of acetyl-TAG, with up to 77 mole percent in the best lines. However, the total oil content was reduced. Analysis of the composition of the acetyl-TAG molecular species using electrospray ionisation mass spectrometry demonstrated the successful synthesis of acetyl-TAG containing MCFAs. Field growth of high-yielding plants generated enough oil for quantification of viscosity. As part of an ongoing design-test-learn cycle, these results, which include not only the synthesis of 'designer' lipids but also their functional analysis, will lead to the future production of such molecules tailored for specific applications.
Membrane topology and identification of key residues of EaDAcT, a plant MBOAT with unusual substrate specificity.[Pubmed: 28715115]
Euonymus alatus diacylglycerol acetyltransferase (EaDAcT) catalyzes the transfer of an acetyl group from acetyl-CoA to the sn-3 position of diacylglycerol to form 3-acetyl-1,2-diacyl-sn-glycerol (acetyl-TAG). EaDAcT belongs to a small, plant-specific subfamily of the membrane bound O-acyltransferases (MBOAT) that acylate different lipid substrates. Sucrose gradient density centrifugation revealed that EaDAcT colocalizes to the same fractions as an endoplasmic reticulum (ER)-specific marker. By mapping the membrane topology of EaDAcT, we obtained an experimentally determined topology model for a plant MBOAT. The EaDAcT model contains four transmembrane domains (TMDs), with both the N- and C-termini orientated toward the lumen of the ER. In addition, there is a large cytoplasmic loop between the first and second TMDs, with the MBOAT signature region of the protein embedded in the third TMD close to the interface between the membrane and the cytoplasm. During topology mapping, we discovered two cysteine residues (C187 and C293) located on opposite sides of the membrane that are important for enzyme activity. In order to identify additional amino acid residues important for acetyltransferase activity, we isolated and characterized acetyltransferases from other acetyl-TAG-producing plants. Among them, the acetyltransferase from Euonymus fortunei possessed the highest activity in vivo and in vitro. Mutagenesis of conserved amino acids revealed that S253, H257, D258 and V263 are essential for EaDAcT activity. Alteration of residues unique to the acetyltransferases did not alter the unique acyl donor specificity of EaDAcT, suggesting that multiple amino acids are important for substrate recognition.
Anti-inflammatory effect of Man-Pen-Fang, a Chinese herbal compound, on chronic pelvic inflammation in rats.[Pubmed: 28652014]
Traditional Chinese Medicine (TCM) has become the focus of research for the treatment of chronic pelvic inflammatory disease (CPID) based on unique medical theory system. Man-Pen-Fang (MPF), a Chinese herbal compound, which is composed of Thlaspi arvense L. (Cruciferae), Gleditsia sinensis Lam. (Leguminosae), Smilax china L. (Liliaceae), Euonymus alatus (Thunb.) Sieb. (Celastraceae) and Vaccaria segetalis (Neck.) (Caryophyllaceae) MPF has been used for the treatment of CPID and exerted significant clinical curative effects. However, the corresponding active principles and anti-inflammatory mechanism of MPF are still unknown.
The protective effect of the active components of ERPC on diabetic peripheral neuropathy in rats.[Pubmed: 28315720]
Euonymus alatus, Radix trichosanthis, Panax notoginseng and Coptis chinensis are popular plants used in traditional Chinese medicine to treat diabetes.
Defining the extreme substrate specificity of Euonymus alatus diacylglycerol acetyltransferase, an unusual membrane bound O-acyltransferase.[Pubmed: 27688773]
Euonymus alatus diacylglycerol acetyltransferase ( Ea DAcT) synthesizes the unusually structured 3-acetyl-1,2-diacylgylycerols (acetyl-TAGs) found in the seeds of a few plant species. A member of the membrane-bound O-acyltransferase (MBOAT) family, Ea DAcT transfers the acetyl group from acetyl-CoA to sn -1,2 diacylglycerol (DAG) to produce acetyl-TAGs. In vitro assays demonstrated that the enzyme is also able to utilize butyryl-CoA and hexanoyl-CoA as acyl donors, though with much less efficiency compared to acetyl-CoA. Acyl-CoAs longer than eight carbons were not used by Ea DAcT. This extreme substrate specificity of Ea DAcT distinguishes it from all other MBOATs which typically catalyze the transfer of much longer acyl groups. In vitro selectivity experiments revealed that Ea DAcT preferentially acetylated DAG molecules containing more double bonds over those with less. However, the enzyme was also able to acetylate saturated DAG containing medium chain fatty acids, albeit with less efficiency. Interestingly, Ea DAcT could only acetylate the free hydroxyl group of sn -1,2 DAG but not the available hydroxyl groups in sn -1,3 DAG or in monoacylglycerols (MAG). Consistent with its similarity to the jojoba wax synthase, Ea DAcT could acetylate fatty alcohols in vitro to produce alkyl acetates. Likewise, when coexpressed in yeast with a fatty acyl-CoA reductase capable of producing fatty alcohols, Ea DAcT was capable of synthesizing alkyl acetates although the efficiency of production was low. This improved understanding of Ea DAcT specificity confirms that the enzyme preferentially utilizes acetyl-CoA to acetylate sn -1,2 DAGs and will be helpful in engineering the production of acetyl-TAGs with improved functionality in transgenic plants.
Euonymus alatus: A Review on Its Phytochemistry and Antidiabetic Activity.[Pubmed: 27642361]
Euonymus alatus (E. alatus) is a medicinal plant used in some Asian countries for treating various conditions including cancer, hyperglycemia, and diabetic complications. This review outlines the phytochemistry and bioactivities of E. alatus related to antidiabetic actions. More than 100 chemical constituents have been isolated and identified from E. alatus, including flavonoids, terpenoids, steroids, lignans, cardenolides, phenolic acids, and alkaloids. Studies in vitro and in vivo have demonstrated the hypoglycemic activity of E. alatus extracts and its certain constituents. The hypoglycemic activity of E. alatus may be related to regulation of insulin signaling and insulin sensitivity, involving PPARγ and aldose reductase pathways. Further studies on E. alatus and its bioactive compounds may help to develop new agents for treating diabetes and diabetic complications.
The Yeast ATF1 Acetyltransferase Efficiently Acetylates Insect Pheromone Alcohols: Implications for the Biological Production of Moth Pheromones.[Pubmed: 26801935]
Many moth pheromones are composed of mixtures of acetates of long-chain (≥10 carbon) fatty alcohols. Moth pheromone precursors such as fatty acids and fatty alcohols can be produced in yeast by the heterologous expression of genes involved in insect pheromone production. Acetyltransferases that subsequently catalyze the formation of acetates by transfer of the acetate unit from acetyl-CoA to a fatty alcohol have been postulated in pheromone biosynthesis. However, so far no fatty alcohol acetyltransferases responsible for the production of straight chain alkyl acetate pheromone components in insects have been identified. In search for a non-insect acetyltransferase alternative, we expressed a plant-derived diacylglycerol acetyltransferase (EaDAcT) (EC 2.3.1.20) cloned from the seed of the burning bush (Euonymus alatus) in a yeast system. EaDAcT transformed various fatty alcohol insect pheromone precursors into acetates but we also found high background acetylation activities. Only one enzyme in yeast was shown to be responsible for the majority of that background activity, the acetyltransferase ATF1 (EC 2.3.1.84). We further investigated the usefulness of ATF1 for the conversion of moth pheromone alcohols into acetates in comparison with Ea DAcT. Overexpression of ATF1 revealed that it was capable of acetylating these fatty alcohols with chain lengths from 10 to 18 carbons with up to 27- and 10-fold higher in vivo and in vitro efficiency, respectively, compared to Ea DAcT. The ATF1 enzyme thus has the potential to serve as the missing enzyme in the reconstruction of the biosynthetic pathway of insect acetate pheromones from precursor fatty acids in yeast.


