AromadendrinCAS# 480-20-6 |
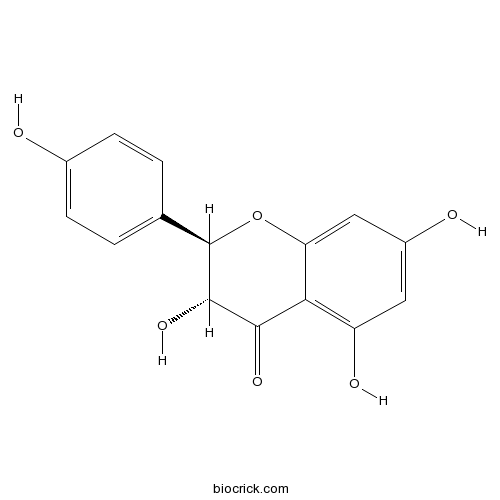
- dihydrokaempferol
Catalog No.:BCC8191
CAS No.:5150-32-3
- (±)-Dihydrokaempferol
Catalog No.:BCX1526
CAS No.:104486-98-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480-20-6 | SDF | Download SDF |
| PubChem ID | 122850 | Appearance | White powder |
| Formula | C15H12O6 | M.Wt | 288.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | (+)-Dihydrokaempferol | ||
| Solubility | Soluble in acetone, chloroform, ethanol and methanol; slightly soluble in water | ||
| Chemical Name | (2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | ||
| SMILES | C1=CC(=CC=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O | ||
| Standard InChIKey | PADQINQHPQKXNL-LSDHHAIUSA-N | ||
| Standard InChI | InChI=1S/C15H12O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,14-18,20H/t14-,15+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Aromadendrin possesses anti-inflammatory, antioxidant, and anti-diabetic properties, it exhibits anti-inflammatory activity through the suppression of nuclear translocation of NF-κB and phosphorylation of JNK in LPS-stimulated RAW 264.7 macrophage cells. 2. Aromadendrin treatment induces adipogenesis by increases in PPARγ2 expression, resulting in stimulation of glucose uptake and ameliorated insulin resistance, suggests that it may represent a potential therapeutic candidate for the management of type 2 DM. 3. Aromadendrin has inhibitory activity on aldose reductase and the formation of advanced glycation end products. |
| Targets | NO | PGE | NOS | COX | NF-kB | JNK | ERK | p38MAPK | PPAR | PI3K | Akt | IkB | IKK |

Aromadendrin Dilution Calculator

Aromadendrin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4686 mL | 17.343 mL | 34.6861 mL | 69.3722 mL | 86.7152 mL |
| 5 mM | 0.6937 mL | 3.4686 mL | 6.9372 mL | 13.8744 mL | 17.343 mL |
| 10 mM | 0.3469 mL | 1.7343 mL | 3.4686 mL | 6.9372 mL | 8.6715 mL |
| 50 mM | 0.0694 mL | 0.3469 mL | 0.6937 mL | 1.3874 mL | 1.7343 mL |
| 100 mM | 0.0347 mL | 0.1734 mL | 0.3469 mL | 0.6937 mL | 0.8672 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Isorhamnetin
Catalog No.:BCN5551
CAS No.:480-19-3
- Taxifolin
Catalog No.:BCN5550
CAS No.:480-18-2
- Morin
Catalog No.:BCN1028
CAS No.:480-16-0
- Izalpinine
Catalog No.:BCN3682
CAS No.:480-14-8
- Oroxylin A
Catalog No.:BCN5363
CAS No.:480-11-5
- Astragalin
Catalog No.:BCN5549
CAS No.:480-10-4
- Eleutherol
Catalog No.:BCN8480
CAS No.:480-00-2
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- NBQX disodium salt
Catalog No.:BCC6907
CAS No.:479347-86-9
- CNQX disodium salt
Catalog No.:BCC6908
CAS No.:479347-85-8
- Orobol
Catalog No.:BCN5553
CAS No.:480-23-9
- Mellein
Catalog No.:BCN4785
CAS No.:480-33-1
- Eugenin
Catalog No.:BCN2921
CAS No.:480-34-2
- Linarin
Catalog No.:BCN5554
CAS No.:480-36-4
- Pinostrobin
Catalog No.:BCN5555
CAS No.:480-37-5
- Pinocembrin
Catalog No.:BCN5556
CAS No.:480-39-7
- Chrysin
Catalog No.:BCN5557
CAS No.:480-40-0
- Naringenin
Catalog No.:BCN5558
CAS No.:480-41-1
- Isosakuranetin
Catalog No.:BCN5559
CAS No.:480-43-3
- Acacetin
Catalog No.:BCN5560
CAS No.:480-44-4
- Hydrangenol
Catalog No.:BCN5561
CAS No.:480-47-7
- Retrorsine
Catalog No.:BCN2119
CAS No.:480-54-6
Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells.[Pubmed:24265867]
Biomol Ther (Seoul). 2013 May 30;21(3):216-21.
Aromadendrin, a flavonol, has been reported to possess a variety of pharmacological activities such as anti-inflammatory, antioxidant, and anti-diabetic properties. However, the underlying mechanism by which Aromadendrin exerts its biological activity has not been extensively demonstrated. The objective of this study is to elucidate the anti-inflammatory mechanism of aromadedrin in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. Aromadendrin significantly suppressed LPS-induced excessive production of pro-inflammatory mediators such as nitric oxide (NO) and PGE2. In accordance, Aromadendrin attenuated LPSinduced overexpression iNOS and COX-2. In addition, Aromadendrin significantly suppressed LPS-induced degradation of IkappaB, which sequesters NF-kappaB in cytoplasm, consequently inhibiting the nuclear translocation of pro-inflammatory transcription factor NF- kappaB. To elucidate the underlying signaling mechanism of anti-inflammatory activity of Aromadendrin, MAPK signaling pathway was examined. Aromadendrin significantly attenuated LPS-induced activation of JNK, but not ERK and p38, in a concentration-dependent manner. Taken together, the present study clearly demonstrates that Aromadendrin exhibits anti-inflammatory activity through the suppression of nuclear translocation of NF-kappaB and phosphorylation of JNK in LPS-stimulated RAW 264.7 macrophage cells.
Stimulation of glucose uptake and improvement of insulin resistance by aromadendrin.[Pubmed:22056597]
Pharmacology. 2011;88(5-6):266-74.
Agents that stimulate glucose uptake and improve insulin resistance may be useful in the management of type 2 diabetes mellitus (DM). Thus, the aims of this study were to assess the effects of Aromadendrin, a flavonoid from Gleditsia sinensis Lam., on stimulation of glucose uptake and improvement of insulin resistance and to characterize the molecular mechanisms underlying these activities. Insulin-stimulated glucose uptake was measured in HepG2 cells and in differentiated 3T3-L1 adipocytes using 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-D-glucose (2-NBDG), a fluorescent D-glucose analog. Expression of the peroxisome proliferator-activated receptor-gamma2 (PPARgamma2) and adipocyte-specific fatty acid binding protein (aP2) mRNAs and the PPARgamma2 protein was analyzed in adipocytes using RT-PCR and immunoblotting, respectively. Insulin-stimulated protein kinase B (Akt/PKB) phosphorylation was measured in high glucose-induced, insulin-resistant HepG2 cells. Similar to 30 mumol/l rosiglitazone, treatment with 30 mumol/l Aromadendrin significantly stimulated insulin-sensitive glucose uptake in both HepG2 cells and 3T3-L1 adipocytes. Aromadendrin treatment also enhanced adipogenesis and caused increases in the expression of PPARgamma2 and aP2 mRNAs and the PPARgamma2 protein in differentiated 3T3-L1 adipocytes. In high glucose-induced, insulin-resistant HepG2 cells, Aromadendrin reversed the inhibition of Akt/PKB phosphorylation in response to insulin, which could be abrogated by pretreatment with LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor. Aromadendrin treatment induced adipogenesis by increases in PPARgamma2 expression, resulting in stimulation of glucose uptake and ameliorated insulin resistance. These findings suggest that Aromadendrin may represent a potential therapeutic candidate for the management of type 2 DM.


