RetrorsineCAS# 480-54-6 |
- Usaramine
Catalog No.:BCN2121
CAS No.:15503-87-4
Quality Control & MSDS
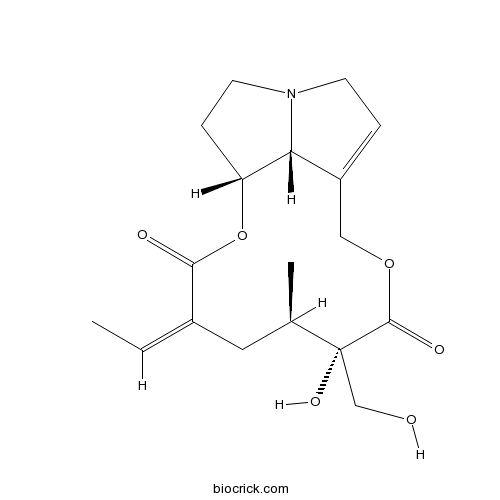
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480-54-6 | SDF | Download SDF |
| PubChem ID | 5281743 | Appearance | Colorless crystals |
| Formula | C18H25NO6 | M.Wt | 351.40 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | β-Longilobine | ||
| Solubility | Soluble in chloroform and methan | ||
| SMILES | CC=C1CC(C(C(=O)OCC2=CCN3C2C(CC3)OC1=O)(CO)O)C | ||
| Standard InChIKey | BCJMNZRQJAVDLD-CQRYIUNCSA-N | ||
| Standard InChI | InChI=1S/C18H25NO6/c1-3-12-8-11(2)18(23,10-20)17(22)24-9-13-4-6-19-7-5-14(15(13)19)25-16(12)21/h3-4,11,14-15,20,23H,5-10H2,1-2H3/b12-3-/t11-,14-,15-,18-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Retrorsine selectively inhibits hepatocyte proliferation and following liver injury evokes small hepatocyte-like progenitor cells. 2. OCT1 mediates the liver-specific uptake of Retrorsine, and plays an important role in Retrorsine-induced hepatotoxicity together with CYP3A4. Consequently, the OCT1 inhibitors could be applied to protect the liver from the toxicity of Retrorsine. |
| Targets | P450 (e.g. CYP17) |

Retrorsine Dilution Calculator

Retrorsine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8458 mL | 14.2288 mL | 28.4576 mL | 56.9152 mL | 71.144 mL |
| 5 mM | 0.5692 mL | 2.8458 mL | 5.6915 mL | 11.383 mL | 14.2288 mL |
| 10 mM | 0.2846 mL | 1.4229 mL | 2.8458 mL | 5.6915 mL | 7.1144 mL |
| 50 mM | 0.0569 mL | 0.2846 mL | 0.5692 mL | 1.1383 mL | 1.4229 mL |
| 100 mM | 0.0285 mL | 0.1423 mL | 0.2846 mL | 0.5692 mL | 0.7114 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Hydrangenol
Catalog No.:BCN5561
CAS No.:480-47-7
- Acacetin
Catalog No.:BCN5560
CAS No.:480-44-4
- Isosakuranetin
Catalog No.:BCN5559
CAS No.:480-43-3
- Naringenin
Catalog No.:BCN5558
CAS No.:480-41-1
- Chrysin
Catalog No.:BCN5557
CAS No.:480-40-0
- Pinocembrin
Catalog No.:BCN5556
CAS No.:480-39-7
- Pinostrobin
Catalog No.:BCN5555
CAS No.:480-37-5
- Linarin
Catalog No.:BCN5554
CAS No.:480-36-4
- Eugenin
Catalog No.:BCN2921
CAS No.:480-34-2
- Mellein
Catalog No.:BCN4785
CAS No.:480-33-1
- Orobol
Catalog No.:BCN5553
CAS No.:480-23-9
- Aromadendrin
Catalog No.:BCN5552
CAS No.:480-20-6
- Lecanoric acid
Catalog No.:BCN5562
CAS No.:480-56-8
- Orsellinic acid
Catalog No.:BCN6574
CAS No.:480-64-8
- 2',4',6'-Trihydroxyacetophenone
Catalog No.:BCN3996
CAS No.:480-66-0
- Jaconine
Catalog No.:BCN2089
CAS No.:480-75-1
- Jacoline
Catalog No.:BCN2088
CAS No.:480-76-2
- Platyphylline
Catalog No.:BCN2115
CAS No.:480-78-4
- Integerrimine
Catalog No.:BCN2131
CAS No.:480-79-5
- Seneciphylline
Catalog No.:BCN5563
CAS No.:480-81-9
- Indicine
Catalog No.:BCN1995
CAS No.:480-82-0
- Echinatine
Catalog No.:BCN1968
CAS No.:480-83-1
- Retronecine
Catalog No.:BCN2034
CAS No.:480-85-3
- Retusine
Catalog No.:BCN2123
CAS No.:480-86-4
Contribution of extrahepatic small cells resembling small hepatocyte-like progenitor cells to liver mass maintenance in transplantation model of retrorsine-pretreated liver.[Pubmed:24083100]
Springerplus. 2013 Sep 8;2:446.
PURPOSE: Retrorsine selectively inhibits hepatocyte proliferation and following liver injury evokes small hepatocyte-like progenitor cells. The aim of this study is to find out whether endogenous extrahepatic cells contribute to small hepatocyte-like progenitor cells after Retrorsine treatment. METHODS: Wild-type Lewis rat liver exposed to Retrorsine was transplanted into GFP transgenic Lewis rat. GFP positive, albumin-producing polygonal cells were expected as reciepient-derived hepatocyte-like cells. RESULTS: Four weeks after transplantation of 50% volume of Retrorsine-pretreated liver, the rate of GFP positive hepatocyte-like cells was 0.02365%. Majority of these cells resided as single cells and their cell size was significantly larger than that of normal hepatocytes (mean cell size; 799.4 um(2) vs. 451.3 um(2), p<0.0001). At eight weeks, clusters of GFP positive small-size albumin-producing cells appeared and occupied 0.00759% of hepatocytes. The morphology of these cells was similar to that of small hepatocyte-like progenitor cells, 12.5% of them were Ki67 positive, majority of them were negative for CYP1A2 staining, and some clusters contained larger cells indicating further maturation. CONCLUSION: Endogenous extrahepatic cells can form a cluster of small cells resembling small hepatocyte-like progenitor cells in a transplanted Retrorsine-pretreated liver. The contribution of extrahepatic cells to liver mass maintenance is quite low and its importance is unclear.
Contribution of mature hepatocytes to small hepatocyte-like progenitor cells in retrorsine-exposed rats with chimeric livers.[Pubmed:23080021]
Hepatology. 2013 Mar;57(3):1215-24.
UNLABELLED: The potential lineage relationship between hepatic oval cells, small hepatocyte-like progenitor cells (SHPCs), and hepatocytes in liver regeneration is debated. To test whether mature hepatocytes can give rise to SHPCs, rats with dipeptidyl peptidase IV (DPPIV) chimeric livers, which harbored endogenous DPPIV-deficient hepatocytes and transplanted DPPIV-positive hepatocytes, were subjected to Retrorsine treatment followed by partial hepatectomy (PH). DPPIV-positive hepatocytes comprised about half of the DPPIV chimeric liver mass. Tissues from DPPIV chimeric livers after Retrorsine/PH treatment showed large numbers of SHPC clusters. None of the SHPC clusters were stained positive for DPPIV in any analyzed samples. Furthermore, serial sections stained for gamma-glutamyl-transpeptidase (GGT, a marker of fetal hepatoblasts) and glucose-6-phosphatase (G6Pase, a marker of mature hepatocytes) showed inverse expression of the two enzymes and a staining pattern consistent with a lineage that begins with GGT(+)/G6Pase(-) to GGT(-)/G6Pase(+) within a single SHPC cluster. Using double immunofluorescence staining for markers specific for hepatic oval cells and hepatocytes in serial sections, oval cell proliferations with CK-19(+)/laminin(+) and OV-6(+)/C/EBP-alpha(-) were shown to extend from periportal areas into the SPHC clusters, differentiating into hepatic lineage by progressive loss of CK-19/laminin expression and appearance of C/EBP-alpha expression towards the cluster side. Cells in the epithelial cell adhesion molecule (EpCAM(+)) SHPC clusters showed membranous EpCAM(+)/HNF-4alpha(+) (hepatocyte nuclear factor-4alpha) staining and were contiguous to the surrounding cytoplasmic EpCAM(+)/HNF-4alpha(-) ductular oval cells. Extensive elimination of oval cell response by repeated administration of 4,4'-methylenedianiline (DAPM) to Retrorsine-exposed rats impaired the emergence of SHPC clusters. CONCLUSION: These findings highly suggest the hepatic oval cells but not mature hepatocytes as the origin of SHPC clusters in Retrorsine-exposed rats.
Disappearance of GFP-positive hepatocytes transplanted into the liver of syngeneic wild-type rats pretreated with retrorsine.[Pubmed:24796859]
PLoS One. 2014 May 5;9(5):e95880.
BACKGROUND AND AIM: Green fluorescent protein (GFP) is a widely used molecular tag to trace transplanted cells in rodent liver injury models. The differing results from various previously reported studies using GFP could be attributed to the immunogenicity of GFP. METHODS: Hepatocytes were obtained from GFP-expressing transgenic (Tg) Lewis rats and were transplanted into the livers of wild-type Lewis rats after they had undergone a partial hepatectomy. The proliferation of endogenous hepatocytes in recipient rats was inhibited by pretreatment with Retrorsine to enhance the proliferation of the transplanted hepatocytes. Transplantation of wild-type hepatocytes into GFP-Tg rat liver was also performed for comparison. RESULTS: All biopsy specimens taken seven days after transplantation showed engraftment of transplanted hepatocytes, with the numbers of transplanted hepatocytes increasing until day 14. GFP-positive hepatocytes in wild-type rat livers were decreased by day 28 and could not be detected on day 42, whereas the number of wild-type hepatocytes steadily increased in GFP-Tg rat liver. Histological examination showed degenerative change of GFP-positive hepatocytes and the accumulation of infiltrating cells on day 28. PCR analysis for the GFP transgene suggested that transplanted hepatocytes were eliminated rather than being retained along with the loss of GFP expression. Both modification of the immunological response using tacrolimus and bone marrow transplantation prolonged the survival of GFP-positive hepatocytes. In contrast, host immunization with GFP-positive hepatocytes led to complete loss of GFP-positive hepatocytes by day 14. CONCLUSION: GFP-positive hepatocytes isolated from GFP-Tg Lewis rats did not survive long term in the livers of Retrorsine-pretreated wild-type Lewis rats. The mechanism underlying this phenomenon most likely involves an immunological reaction against GFP. The influence of GFP immunogenicity on cell transplantation models should be considered in planning in vivo experiments using GFP and in interpreting their results.
Involvement of organic cation transporter 1 and CYP3A4 in retrorsine-induced toxicity.[Pubmed:24799337]
Toxicology. 2014 Aug 1;322:34-42.
Retrorsine (RTS) is a hepatotoxic pyrrolizidine alkaloid present in plants of the Senecio genus. The present study is aimed at clarifying the role of organic cation transporters (OCTs) in the liver disposition of RTS, and the coupling of OCT1 and cytochrome P450 (CYP) 3A4 in the hepatotoxicity of RTS. MDCK or LLC-PK1 cells stably expressing liver uptake or efflux transporters were used to investigate the interaction of RTS with these transporters. Primary cultured rat hepatocytes (PCRH) and double-transfected MDCK-hOCT1-CYP3A4 cells were used to determine the contribution of OCT1 and CYP3A4 to the toxicity of RTS. The results showed that RTS inhibited the OCT1-mediated 1-methyl-4-phenylpyridinium (MPP(+)) uptake in MDCK-hOCT1 cells with the IC50 of 2.25+/-0.30muM. The uptake of RTS in MDCK-hOCT1 cells and PCRH was significantly inhibited by OCT1 inhibitors, while hOCT3, human multidrug and toxin extrusion (hMATE) transporter 1, multidrug resistance 1 (MDR1), and breast cancer resistance protein (BCRP) showed weak or no obvious interaction with RTS. The toxic effect of RTS on the PCRH was attenuated by OCT1 inhibitors, quinidine and (+)-tetrahydropalmatine ((+)-THP). Compared to mock cells, MDCK-CYP3A4 cells showed a decrease in viability after being treated with RTS. Furthermore, RTS showed a more severe toxicity in the OCT1/CYP3A4 double-transfected cells compared to all other cells. Our data suggests that OCT1 mediates the liver-specific uptake of RTS, and plays an important role in RTS-induced hepatotoxicity together with CYP3A4. Consequently, the OCT1 inhibitors could be applied to protect the liver from the toxicity of RTS.
Repopulation of the immunosuppressed retrorsine-treated infant rat liver with human hepatocytes.[Pubmed:23683097]
Xenotransplantation. 2013 Jul-Aug;20(4):227-38.
BACKGROUND: We previously generated humanized chimeric mice by transplanting h-hepatocytes into the livers of the diseased-liver transgenic mouse model with immunodeficient background. These mice with livers mostly replaced by human (h) hepatocytes have been proved to be useful for research on drug metabolism and toxicity and on intrahepatic pathogens such as hepatitis. However, their small body size prohibited collecting sufficient biological samples and made surgical manipulation difficult, which motivated us to produce humanized larger animal(s) bearing h-hepatocytes. METHODS: Fischer 344 (F344) rats at 2 weeks of age were administrated with hepatotoxin Retrorsine (RS) and then transplanted with syngeneic F344 rat (r)- or h-hepatocytes via the portal vein. The hosts were injected daily with FK506 immunosuppressant. The livers were harvested periodically for determining donor-cell replacement ratios and compared with those of the humanized chimeric mice, and liver-specific mRNA and protein expressions by immunohistochemistry and reverse-transcription PCR. RESULTS: RS treatment of infant rats inhibited hepatocyte proliferation, resulting in decreased liver weight and megalocytic changes in hepatocytes. R-hepatocytes transplanted into RS-treated rats engrafted into and repopulated the liver at ratios of 16.4 +/- 6.7% and 48.3 +/- 29.3% at 3 and 6 weeks after transplantation, respectively. H-hepatocytes also engrafted into the rat liver and showed a repopulation ratio of 2.5 +/- 1.5% at 3 weeks post-transplantation, which was comparable to the ratio in the humanized chimeric mouse model at least until 3 weeks. Propagated h-hepatocytes in the rat liver expressed hepatocyte-specific mRNA and proteins at least 3 weeks after transplantation. CONCLUSIONS: Xenogeneic hepatocytes were able to engraft rat liver and grow well therein for at least 3 weeks post-transplantation in rats when immunosuppression was combined appropriately with liver injury at comparable levels to the well-characterized humanized chimeric mouse model.


