MelleinCAS# 480-33-1 |
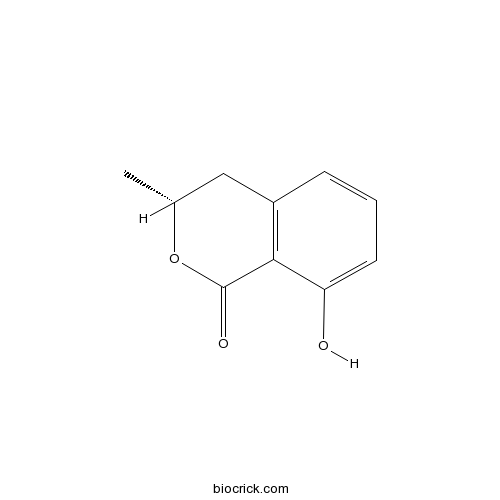
- (+)-Mellein
Catalog No.:BCN7220
CAS No.:62623-84-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480-33-1 | SDF | Download SDF |
| PubChem ID | 114679 | Appearance | Powder |
| Formula | C10H10O3 | M.Wt | 178.2 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3R)-8-hydroxy-3-methyl-3,4-dihydroisochromen-1-one | ||
| SMILES | CC1CC2=C(C(=CC=C2)O)C(=O)O1 | ||
| Standard InChIKey | KWILGNNWGSNMPA-ZCFIWIBFSA-N | ||
| Standard InChI | InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3/t6-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Standard reference |
| In vitro | An in planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum.[Pubmed: 25326302 ]Appl Environ Microbiol. 2015 Jan;81(1):177-86.Parastagonospora nodorum is a pathogen of wheat that affects yields globally.
Synthesis of (R)-mellein by a partially reducing iterative polyketide synthase.[Pubmed: 22793256]J Am Chem Soc. 2012 Jul 25;134(29):11924-7.Disruption of the corresponding SN477 gene resulted in the loss of production of two compounds, which we identified as (R)-Mellein and (R)-O-methylMellein. |

Mellein Dilution Calculator

Mellein Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.6117 mL | 28.0584 mL | 56.1167 mL | 112.2334 mL | 140.2918 mL |
| 5 mM | 1.1223 mL | 5.6117 mL | 11.2233 mL | 22.4467 mL | 28.0584 mL |
| 10 mM | 0.5612 mL | 2.8058 mL | 5.6117 mL | 11.2233 mL | 14.0292 mL |
| 50 mM | 0.1122 mL | 0.5612 mL | 1.1223 mL | 2.2447 mL | 2.8058 mL |
| 100 mM | 0.0561 mL | 0.2806 mL | 0.5612 mL | 1.1223 mL | 1.4029 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Orobol
Catalog No.:BCN5553
CAS No.:480-23-9
- Aromadendrin
Catalog No.:BCN5552
CAS No.:480-20-6
- Isorhamnetin
Catalog No.:BCN5551
CAS No.:480-19-3
- Taxifolin
Catalog No.:BCN5550
CAS No.:480-18-2
- Morin
Catalog No.:BCN1028
CAS No.:480-16-0
- Izalpinine
Catalog No.:BCN3682
CAS No.:480-14-8
- Oroxylin A
Catalog No.:BCN5363
CAS No.:480-11-5
- Astragalin
Catalog No.:BCN5549
CAS No.:480-10-4
- Eleutherol
Catalog No.:BCN8480
CAS No.:480-00-2
- CP-809101
Catalog No.:BCC1498
CAS No.:479683-64-2
- 1-O-galloyl-2-O-cinnamoyl-beta-d-glucose
Catalog No.:BCC3967
CAS No.:
- AUDA
Catalog No.:BCC4023
CAS No.:479413-70-2
- Eugenin
Catalog No.:BCN2921
CAS No.:480-34-2
- Linarin
Catalog No.:BCN5554
CAS No.:480-36-4
- Pinostrobin
Catalog No.:BCN5555
CAS No.:480-37-5
- Pinocembrin
Catalog No.:BCN5556
CAS No.:480-39-7
- Chrysin
Catalog No.:BCN5557
CAS No.:480-40-0
- Naringenin
Catalog No.:BCN5558
CAS No.:480-41-1
- Isosakuranetin
Catalog No.:BCN5559
CAS No.:480-43-3
- Acacetin
Catalog No.:BCN5560
CAS No.:480-44-4
- Hydrangenol
Catalog No.:BCN5561
CAS No.:480-47-7
- Retrorsine
Catalog No.:BCN2119
CAS No.:480-54-6
- Lecanoric acid
Catalog No.:BCN5562
CAS No.:480-56-8
- Orsellinic acid
Catalog No.:BCN6574
CAS No.:480-64-8
Synthesis of (R)-mellein by a partially reducing iterative polyketide synthase.[Pubmed:22793256]
J Am Chem Soc. 2012 Jul 25;134(29):11924-7.
Mellein and the related 3,4-dihydroisocoumarins are a family of natural products with interesting biological properties. The mechanisms of dihydroisocoumarin biosynthesis remain largely speculative today. Here we report the synthesis of Mellein by a partially reducing iterative polyketide synthase (PR-PKS) as a pentaketide product. Remarkably, despite the head-to-tail homology shared with several fungal and bacterial PR-PKSs, the Mellein synthase exhibits a distinct keto reduction pattern in the synthesis of the pentaketide. We present evidence to show that the ketoreductase (KR) domain alone is able to recognize and differentiate the polyketide intermediates, which provides a mechanistic explanation for the programmed keto reduction in these PR-PKSs.
An in planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum.[Pubmed:25326302]
Appl Environ Microbiol. 2015 Jan;81(1):177-86.
Parastagonospora nodorum is a pathogen of wheat that affects yields globally. Previous transcriptional analysis identified a partially reducing polyketide synthase (PR-PKS) gene, SNOG_00477 (SN477), in P. nodorum that is highly upregulated during infection of wheat leaves. Disruption of the corresponding SN477 gene resulted in the loss of production of two compounds, which we identified as (R)-Mellein and (R)-O-methylMellein. Using a Saccharomyces cerevisiae yeast heterologous expression system, we successfully demonstrated that SN477 is the only enzyme required for the production of (R)-Mellein. This is the first identification of a fungal PKS that is responsible for the synthesis of (R)-Mellein. The P. nodorum DeltaSN477 mutant did not show any significant difference from the wild-type strain in its virulence against wheat. However, (R)-Mellein at 200 mug/ml inhibited the germination of wheat (Triticum aestivum) and barrel medic (Medicago truncatula) seeds. Comparative sequence analysis identified the presence of Mellein synthase (MLNS) homologues in several Dothideomycetes and two sodariomycete genera. Phylogenetic analysis suggests that the MLNSs in fungi and bacteria evolved convergently from fungal and bacterial 6-methylsalicylic acid synthases.


