RetronecineCAS# 480-85-3 |
- Heliotridine
Catalog No.:BCN8929
CAS No.:520-63-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480-85-3 | SDF | Download SDF |
| PubChem ID | 10198 | Appearance | White powder |
| Formula | C8H13NO2 | M.Wt | 155.20 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethan | ||
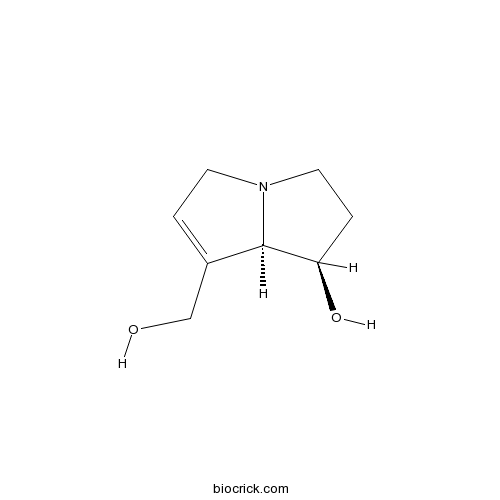
| Chemical Name | (1R,8R)-7-(hydroxymethyl)-2,3,5,8-tetrahydro-1H-pyrrolizin-1-ol | ||
| SMILES | C1CN2CC=C(C2C1O)CO | ||
| Standard InChIKey | HJSJELVDQOXCHO-HTQZYQBOSA-N | ||
| Standard InChI | InChI=1S/C8H13NO2/c10-5-6-1-3-9-4-2-7(11)8(6)9/h1,7-8,10-11H,2-5H2/t7-,8-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Retronecine can be metabolized to form DHP by rat liver microsomal enzymes and interacts with DNA to produce DHP-DNA adducts and Retronecine N-oxide undergoes the biotransformation to the parent compound, Retronecine. 2. Retronecine acts as the better competitor for the competitive inhibition of antibodies to Retronecine. |

Retronecine Dilution Calculator

Retronecine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4433 mL | 32.2165 mL | 64.433 mL | 128.866 mL | 161.0825 mL |
| 5 mM | 1.2887 mL | 6.4433 mL | 12.8866 mL | 25.7732 mL | 32.2165 mL |
| 10 mM | 0.6443 mL | 3.2216 mL | 6.4433 mL | 12.8866 mL | 16.1082 mL |
| 50 mM | 0.1289 mL | 0.6443 mL | 1.2887 mL | 2.5773 mL | 3.2216 mL |
| 100 mM | 0.0644 mL | 0.3222 mL | 0.6443 mL | 1.2887 mL | 1.6108 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Echinatine
Catalog No.:BCN1968
CAS No.:480-83-1
- Indicine
Catalog No.:BCN1995
CAS No.:480-82-0
- Seneciphylline
Catalog No.:BCN5563
CAS No.:480-81-9
- Integerrimine
Catalog No.:BCN2131
CAS No.:480-79-5
- Platyphylline
Catalog No.:BCN2115
CAS No.:480-78-4
- Jacoline
Catalog No.:BCN2088
CAS No.:480-76-2
- Jaconine
Catalog No.:BCN2089
CAS No.:480-75-1
- 2',4',6'-Trihydroxyacetophenone
Catalog No.:BCN3996
CAS No.:480-66-0
- Orsellinic acid
Catalog No.:BCN6574
CAS No.:480-64-8
- Lecanoric acid
Catalog No.:BCN5562
CAS No.:480-56-8
- Retrorsine
Catalog No.:BCN2119
CAS No.:480-54-6
- Hydrangenol
Catalog No.:BCN5561
CAS No.:480-47-7
- Retusine
Catalog No.:BCN2123
CAS No.:480-86-4
- Dicrotaline
Catalog No.:BCN2079
CAS No.:480-87-5
- Benzofuroxan
Catalog No.:BCC8852
CAS No.:480-96-6
- Carbenicillin, Disodium Salt
Catalog No.:BCC1200
CAS No.:4800-94-6
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Lucialdehyde B
Catalog No.:BCN2450
CAS No.:480439-84-7
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Edoxaban tosylate
Catalog No.:BCC1544
CAS No.:480449-71-6
- Alpha-Santonin
Catalog No.:BCN7828
CAS No.:481-06-1
- alpha-Spinasterol
Catalog No.:BCN5564
CAS No.:481-18-5
- Epiandrosterone
Catalog No.:BCC4481
CAS No.:481-29-8
- Ecgonine
Catalog No.:BCN1907
CAS No.:481-37-8
Metabolic activation of retronecine and retronecine N-oxide - formation of DHP-derived DNA adducts.[Pubmed:18842697]
Toxicol Ind Health. 2008 Apr;24(3):181-8.
We have previously reported that metabolism of a series of pyrrolizidine alkaloids in vitro and in vivo generated a set of (+/-)6,7-dihydro-7-hydroxy-1-hydroxymethyl-5H-pyrrolizine (DHP)-derived DNA adducts. It has also been shown that the levels of the DHP-derived DNA adduct formation correlated closely with the tumorigenic potencies of the mice fed with different doses of riddelliine. Retronecine is the necine base and the structurally smallest chemical of the Retronecine-type pyrrolizidine alkaloids. Although it has been reported that microsomal metabolism of Retronecine generated DHP as a metabolite, it was yet not known whether metabolism of Retronecine in vivo could generate DHP-derived DNA adducts and if formed, whether or not the levels of DNA adducts were comparable with those formed from the other tumorigenic Retronecine-type pyrrolizidine alkaloids, such as riddelliine, retrorsine, and monocrotaline. In this investigation, the in-vitro and in-vivo metabolic activation of Retronecine was studied. Rat liver microsomal metabolism of Retronecine in the presence of calf thymus DNA resulted in the formation of a set of DHP-DNA adducts. The metabolism of Retronecine N-oxide under similar conditions also formed the similar set of DHP-DNA adducts. The level of DNA adducts from Retronecine was enhanced when metabolism by liver microsomes from phenobarbital (PB)-induced rats were used. The DHP-DNA adducts were also found in the liver DNA of female F344 rats treated with Retronecine or Retronecine N-oxide. The highest level of the total DHP-DNA adducts was found in liver DNA from the rats treated with dehydroRetronecine (DHR). The order of the levels of DNA adducts in the liver DNA samples from rats treated with various pyrrolizidine alkaloids was: DHR > riddelliine > riddelliine N-oxide >> Retronecine > Retronecine N-oxide. The results indicate that 1) Retronecine can be metabolized to form DHP by rat liver microsomal enzymes and interacts with DNA to produce DHP-DNA adducts and 2) Retronecine N-oxide undergoes the biotransformation to the parent compound, Retronecine. The results from this and our previous findings strongly suggest that formation of DHP-DNA adducts may be a potential biomarker for pyrrolizidine alkaloid carcinogenesis.
Further evidence on the intramolecular lactonization in rat liver microsomal metabolism of 12-O-acetylated retronecine-type pyrrolizidine alkaloids.[Pubmed:24427937]
Nat Prod Commun. 2013 Nov;8(11):1545-6.
We have previously found evidence of intramolecular lactonization in rat liver microsomal metabolism of isoline, a 12-O-acetylated pyrrolizidine alkaloid. In this study, the metabolism of another 12-O-acetylated pyrrolizidine alkaloid, acetylduciformine, by the proposed transformation pathway was investigated under the same incubation conditions. Two deacetylated metabolites from acetylduciformine were isolated and purified by chromatographic methods, and further characterized based on their physical properties and spectral data. One metabolite (lankongensisine A) was the lactone of another one (duciformine). Both compounds were first obtained as hydrolyzed metabolites from acetylduciformine by rat liver microsomes. More importantly, the present study provided further evidence for the intramolecular lactonization in the microsomal metabolism of 12-O-acetylated Retronecine-type PAs.
A competitive enzyme-linked immunosorbent assay (ELISA) to detect retronecine and monocrotaline in vitro.[Pubmed:2508272]
Toxicon. 1989;27(9):1059-64.
Antibodies to the nonesterified pyrrolizidine nucleus, Retronecine (155 mol.wt), were produced in rabbits and detected using an avidin-biotin antibody ELISA. A competitive ELISA for the detection of Retronecine and the cyclic diester monocrotaline was also developed using the antiserum produced against the hapten conjugate, Retronecine-bovine serum albumin. Retronecine was obtained by hydrolysis of monocrotaline, succinylated and directly coupled to bovine serum albumin or ovalbumin. Antibodies to the pyrrolizidine nucleus, Retronecine, can be detected within 5 min after the addition of substrate using the avidin-biotin ELISA. Competitive inhibition of antibodies to Retronecine is obtained by the addition of known amounts (0-11.42 micrograms/microliters) of either the homologous antigen, Retronecine, or the heterologous antigen, monocrotaline, however, Retronecine acts as the better competitor.


