Edoxaban tosylateFxa inhibitor CAS# 480449-71-6 |
- Bivalirudin Trifluoroacetate
Catalog No.:BCC1421
CAS No.:128270-60-0
- Dabigatran ethyl ester
Catalog No.:BCC1512
CAS No.:429658-95-7
- 5-R-Rivaroxaban
Catalog No.:BCC1313
CAS No.:865479-71-6
- Dabigatran etexilate mesylate
Catalog No.:BCC1511
CAS No.:872728-81-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 480449-71-6 | SDF | Download SDF |
| PubChem ID | 44610678 | Appearance | Powder |
| Formula | C31H38ClN7O7S2 | M.Wt | 720.26 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DU-176b | ||
| Solubility | 25℃: DMSO | ||
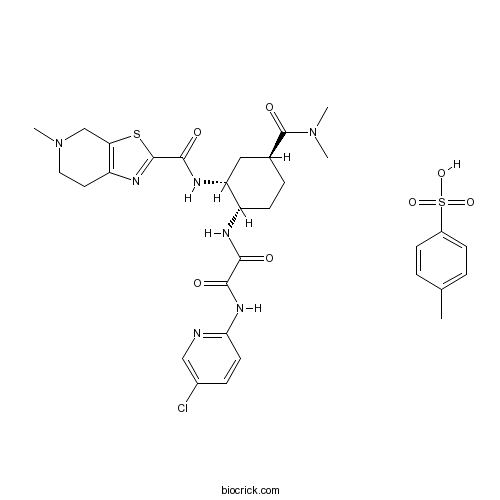
| Chemical Name | N'-(5-chloropyridin-2-yl)-N-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-[(5-methyl-6,7-dihydro-4H-[1,3]thiazolo[5,4-c]pyridine-2-carbonyl)amino]cyclohexyl]oxamide;4-methylbenzenesulfonic acid | ||
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)O.CN1CCC2=C(C1)SC(=N2)C(=O)NC3CC(CCC3NC(=O)C(=O)NC4=NC=C(C=C4)Cl)C(=O)N(C)C | ||
| Standard InChIKey | ZLFZITWZOYXXAW-QXXZOGQOSA-N | ||
| Standard InChI | InChI=1S/C24H30ClN7O4S.C7H8O3S/c1-31(2)24(36)13-4-6-15(27-20(33)21(34)30-19-7-5-14(25)11-26-19)17(10-13)28-22(35)23-29-16-8-9-32(3)12-18(16)37-23;1-6-2-4-7(5-3-6)11(8,9)10/h5,7,11,13,15,17H,4,6,8-10,12H2,1-3H3,(H,27,33)(H,28,35)(H,26,30,34);2-5H,1H3,(H,8,9,10)/t13-,15-,17+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Edoxaban(DU-176) is an oral factor Xa (FXa) inhibitor in clinical development for stroke prevention
IC50 Value:
Target: factor Xa
Edoxaban is an oral factor Xa (FXa) inhibitor in clinical development for stroke prevention in patients with atrial fibrillation, an elderly population that frequently receives aspirin (ASA) and/or nonsteroidal anti-inflammatory drugs for concurrent illnesses[1].
in vitro: Edoxaban PK was not affected by concomitant low-dose ASA or naproxen, but high-dose ASA increased systemic exposure of edoxaban by approximately 30%. The effects of edoxaban on prothrombin time, activated partial thromboplastin time, international normalized ratio, anti-FXa, and intrinsic FXa activity were not influenced by administration with ASA or naproxen. Inhibition of platelet aggregation by high-dose ASA, low-dose ASA, or naproxen was not affected by edoxaban[1].
in vivo: Forty-eight subjects, aged 18 to 45 years, received either edoxaban 60 mg once daily × 7 days (n = 24) or digoxin 0.25 mg twice daily × 2 days and once daily × 5 days (n = 24) and then concomitantly for 7 days. Serial blood and urine samples were collected for digoxin and edoxaban concentrations on days 7 and 14. Serial coagulation assays were measured for edoxaban on days 7 and 14. Edoxaban PK parameters demonstrated mild increases in area under the curve and peak concentrations of 9.5% and 15.6%, respectively[2],
Clinical trial: Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans was reported[3]. References: | |||||

Edoxaban tosylate Dilution Calculator

Edoxaban tosylate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.3884 mL | 6.9419 mL | 13.8839 mL | 27.7678 mL | 34.7097 mL |
| 5 mM | 0.2777 mL | 1.3884 mL | 2.7768 mL | 5.5536 mL | 6.9419 mL |
| 10 mM | 0.1388 mL | 0.6942 mL | 1.3884 mL | 2.7768 mL | 3.471 mL |
| 50 mM | 0.0278 mL | 0.1388 mL | 0.2777 mL | 0.5554 mL | 0.6942 mL |
| 100 mM | 0.0139 mL | 0.0694 mL | 0.1388 mL | 0.2777 mL | 0.3471 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50 Value:N/A Edoxaban is an oral factor Xa (FXa) inhibitor in clinical development for stroke prevention in patients with atrial fibrillation, an elderly population that frequently receives aspirin (ASA) and/or nonsteroidal anti-inflammatory drugs for concurrent illnesses[1]. in vitro: Edoxaban PK was not affected by concomitant low-dose ASA or naproxen, but high-dose ASA increased systemic exposure of edoxaban by approximately 30%. The effects of edoxaban on prothrombin time, activated partial thromboplastin time, international normalized ratio, anti-FXa, and intrinsic FXa activity were not influenced by administration with ASA or naproxen. Inhibition of platelet aggregation by high-dose ASA, low-dose ASA, or naproxen was not affected by edoxaban[1]. in vivo: Forty-eight subjects, aged 18 to 45 years, received either edoxaban 60 mg once daily × 7 days (n = 24) or digoxin 0.25 mg twice daily × 2 days and once daily × 5 days (n = 24) and then concomitantly for 7 days. Serial blood and urine samples were collected for digoxin and edoxaban concentrations on days 7 and 14. Serial coagulation assays were measured for edoxaban on days 7 and 14. Edoxaban PK parameters demonstrated mild increases in area under the curve and peak concentrations of 9.5% and 15.6%, respectively[2], Clinical trial: Pharmacokinetics, biotransformation, and mass balance of edoxaban, a selective, direct factor Xa inhibitor, in humans was reported[3].
- Edoxaban
Catalog No.:BCC1543
CAS No.:480449-70-5
- Lucialdehyde B
Catalog No.:BCN2450
CAS No.:480439-84-7
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Carbenicillin, Disodium Salt
Catalog No.:BCC1200
CAS No.:4800-94-6
- Benzofuroxan
Catalog No.:BCC8852
CAS No.:480-96-6
- Dicrotaline
Catalog No.:BCN2079
CAS No.:480-87-5
- Retusine
Catalog No.:BCN2123
CAS No.:480-86-4
- Retronecine
Catalog No.:BCN2034
CAS No.:480-85-3
- Echinatine
Catalog No.:BCN1968
CAS No.:480-83-1
- Indicine
Catalog No.:BCN1995
CAS No.:480-82-0
- Seneciphylline
Catalog No.:BCN5563
CAS No.:480-81-9
- Integerrimine
Catalog No.:BCN2131
CAS No.:480-79-5
- Alpha-Santonin
Catalog No.:BCN7828
CAS No.:481-06-1
- alpha-Spinasterol
Catalog No.:BCN5564
CAS No.:481-18-5
- Epiandrosterone
Catalog No.:BCC4481
CAS No.:481-29-8
- Ecgonine
Catalog No.:BCN1907
CAS No.:481-37-8
- Juglone
Catalog No.:BCN2639
CAS No.:481-39-0
- Plumbagin
Catalog No.:BCN2586
CAS No.:481-42-5
- Ginkgetin
Catalog No.:BCN2319
CAS No.:481-46-9
- Cepharanthine
Catalog No.:BCN5393
CAS No.:481-49-2
- Tangeretin
Catalog No.:BCN2386
CAS No.:481-53-8
- Aloeemodin
Catalog No.:BCN5565
CAS No.:481-72-1
- Citreorosein
Catalog No.:BCN5566
CAS No.:481-73-2
- Chrysophanol
Catalog No.:BCN5567
CAS No.:481-74-3
Edoxaban tosylate.[Pubmed:21446778]
Am J Cardiovasc Drugs. 2011;11(2):129-35.
Daiichi Sankyo is developing Edoxaban tosylate (DU 176b; DU-176; DU-176b; DU176b) as an orally active direct factor Xa inhibitor for the prevention of stroke and the prevention and treatment of venous thromboembolism. Two dosing regimens of Edoxaban tosylate are being compared with warfarin over 24 months in the ENGAGE AF TIMI 48 trial (NCT00781391) in over 21 000 patients with atrial fibrillation in North and South America, Africa, Asia, Europe, Australia, and New Zealand. Edoxaban tosylate is also being compared with warfarin in the treatment and prevention of recurrent thromboembolic events in approximately 7500 patients with deep-vein thrombosis and/or pulmonary embolism in the HOKUSAI VTE trial (NCT00986154) being conducted in 40 countries. This review discusses the development history and scientific profile of this new compound.


