Rehmannia glutinosa
Rehmannia glutinosa
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.

Natural products/compounds from Rehmannia glutinosa
- Cat.No. Product Name CAS Number COA
-
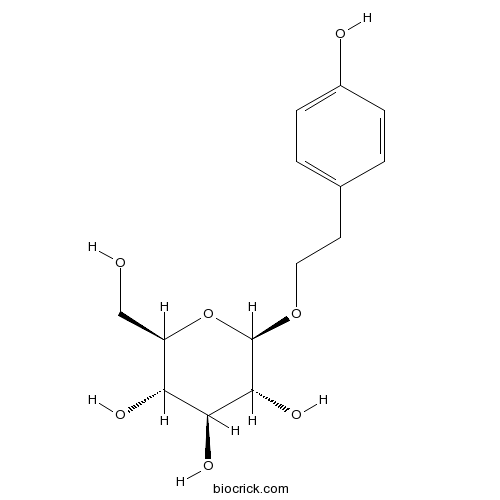
BCN5966
Salidroside10338-51-9
Instructions
-
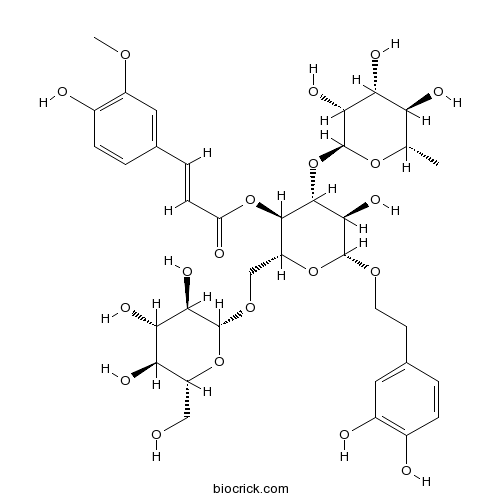
BCN2858
Jionoside B1120406-37-3
Instructions

-
BCN2922
Jionoside A1120444-60-2
Instructions
-
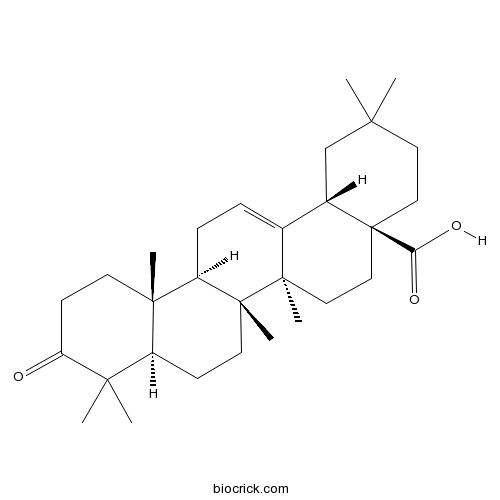
BCN3171
3-oxo-Olean-12-en-28-oic acid17990-42-0
Instructions
-
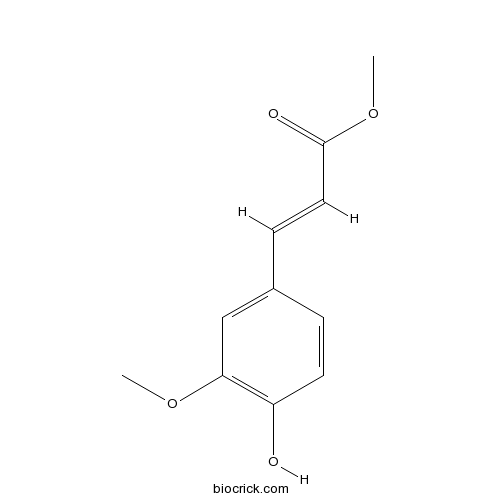
BCN9073
Ferulic Acid Methyl Ester2309-07-1
Instructions
-
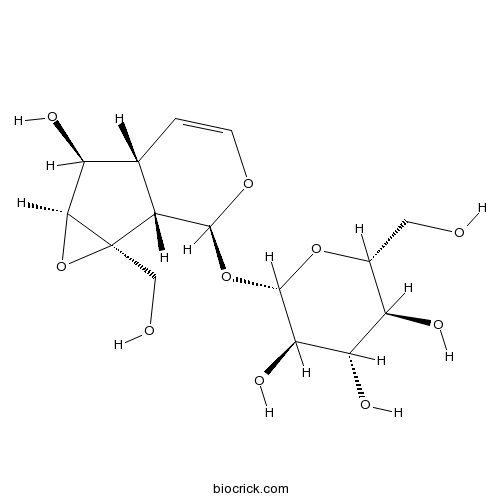
BCN5094
Catalpol2415-24-9
Instructions
-
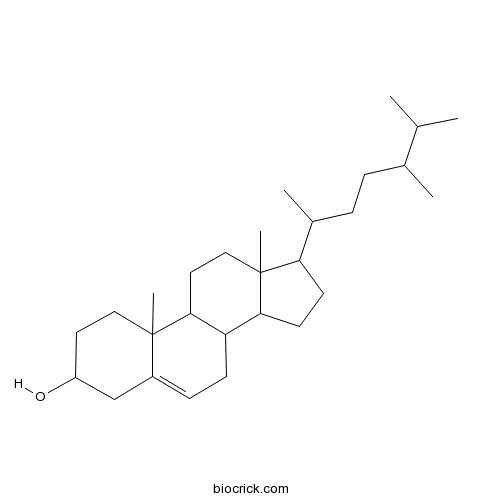
BCN3181
Campesterol474-62-4
Instructions
-
BCN5355
Aucubin479-98-1
Instructions

-
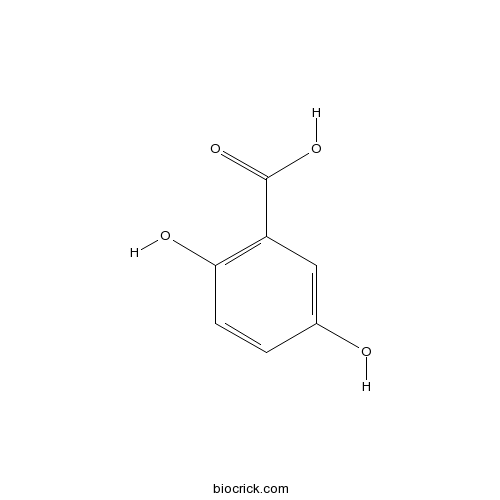
BCN3408
Gentisic acid490-79-9
Instructions
-
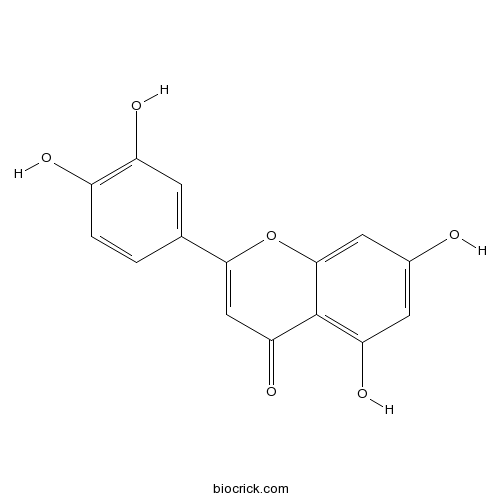
BCN5600
Luteolin491-70-3
Instructions
-
BCN5608
2-(4-Hydroxyphenyl)ethanol501-94-0
Instructions
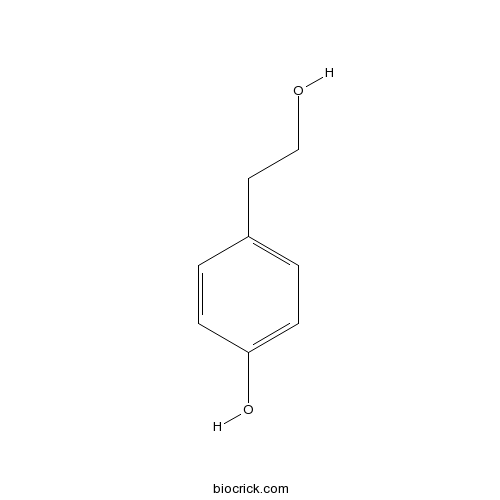
-
BCN5616
Oleanolic acid508-02-1
Instructions

-
BCN8427
Raffinose512-69-6
Instructions
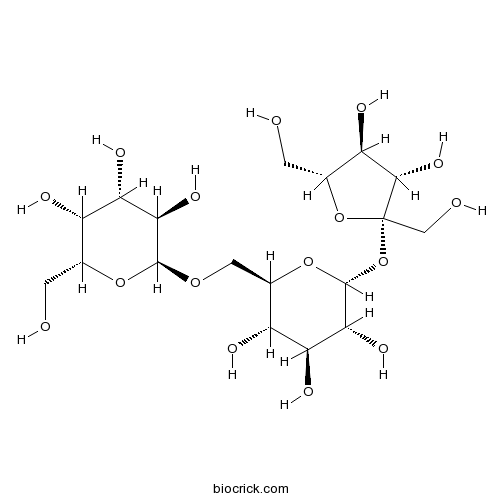
-
BCN2883
Ajugol52949-83-4
Instructions
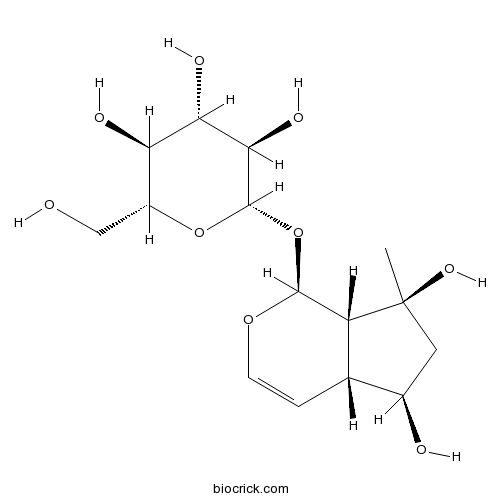
-
BCN5796
Adenosine58-61-7
Instructions
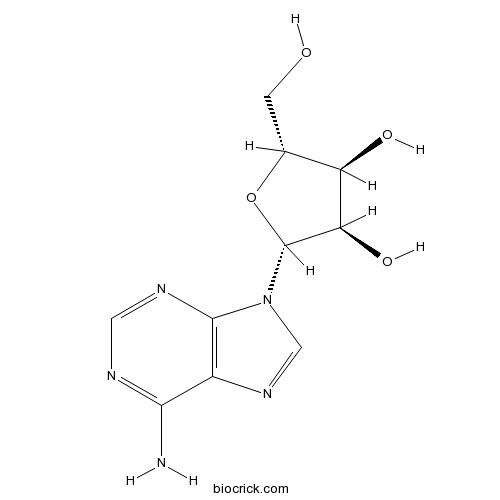
-
BCN4090
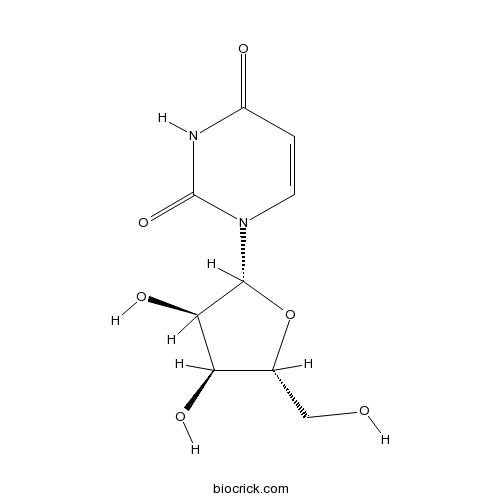
Uridine58-96-8
Instructions
-
BCN4136
Acteoside61276-17-3
Instructions

-
BCN4137
Isoacteoside61303-13-7
Instructions

-
BCC4450
Adenine73-24-5
Instructions

-
BCN4327
Ursolic acid77-52-1
Instructions

-
BCN2885
Rehmannioside A81720-05-0
Instructions

-
BCN2362
Rhmannioside D81720-08-3
Instructions

-
BCN4953
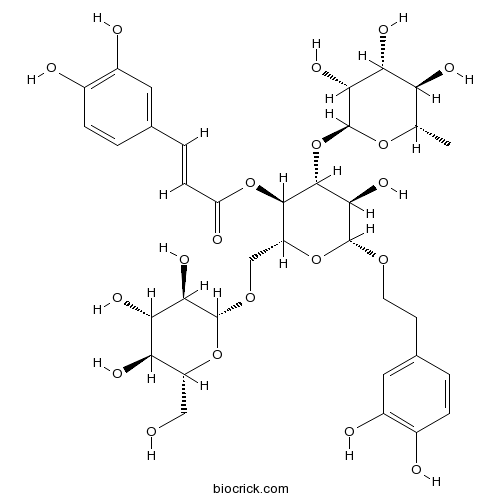
Echinacoside82854-37-3
Instructions
-
BCN1015
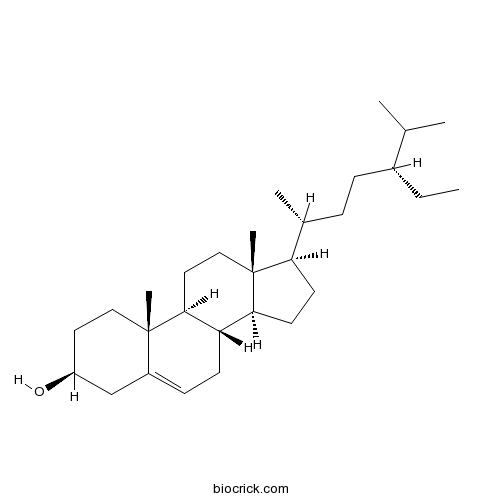
Beta-Sitosterol83-46-5
Instructions
-
BCN2668
Cistanoside A93236-42-1
Instructions

-
BCN4537
3,4-Dihydroxybenzoic acid99-50-3
Instructions
-
BCN4546
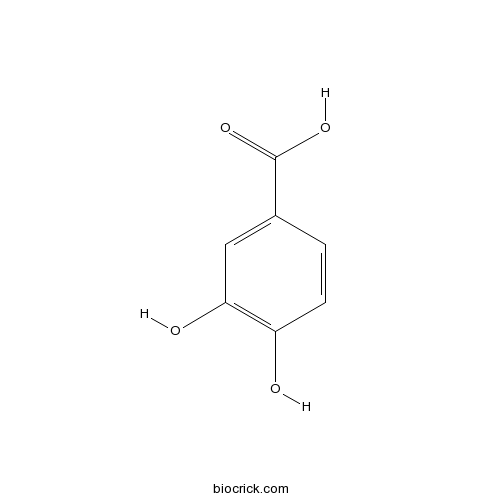
4-Hydroxybenzoic acid99-96-7
Instructions

Rhizosphere fungal community dynamics associated with Rehmannia glutinosa replant disease in a consecutive monoculture regime.[Pubmed: 29975158]
Consecutive monoculture of Rehmannia glutinosa in the same field leads to a severe decline in both quality and yield of tuberous roots, the most useful part in traditional Chinese medicine. Fungi are an important and diverse group of microorganisms in the soil ecosystem and play crucial roles towards soil health. In this study, high-throughput pyrosequencing of ITS2 rDNA amplicons was applied to gain insight into how consecutive monoculture practice influence and stimulate R. glutinosa rhizosphere and bulk soil fungal communities. The results from non-metric multidimensional scaling ordination and clustering analysis revealed distinctive differences between rhizosphere and bulk soil fungal communities. However, longer-term monocultured bulk soils were more similar to the rhizosphere soils in comparison to the shorter-term monocultured bulk soils. Moreover, consecutive monoculture caused a gradual shift in the composition and structure of the soil fungal community. The cultivation of this plant led to the appearance of some exclusive OTUs in rhizosphere or bulk soils that were assigned to Fusarium, Rhizoctonia, etc. Furthermore, the sum of the relative abundances of Fusarium, Cylindrocarpon, Gibberella (belonging to Nectriaceae), Rhizoctonia, Thanatephorus, Ceratobasidium (belonging to Ceratobasidiaceae) and Lectera, Plectosporium (belonging to Plectosphaerellaceae) was significantly higher in the consecutively monocultured (CM) than in the newly planted (NP) in both rhizosphere and bulk soils. In particular, Fusarium was significantly higher in CM than in NP in rhizosphere, and higher in rhizosphere soils than in bulk soils for each treatment. Pathogenicity test showed that both Fusarium strains isolated were pathogenic to R. glutinosa seedlings. In addition, the culture filtrate and mycotoxins produced by F. oxysporum significantly repressed the growth of the antagonistic bacterium, Pseudomonas aeruginosa. In conclusion, consecutive monoculture of R. glutinosa restructured the fungal communities in both rhizosphere and bulk soils, but bulk effects developed more slowly over time in comparison to rhizosphere effects. Furthermore, microbial interactions might lead to a reduction in the abundance of beneficial microbes.
Catalpol inhibits migration and induces apoptosis in gastric cancer cells and in athymic nude mice.[Pubmed: 29864961]
Gastric cancer is one of the leading factors, causing tumor-associated death worldwide. However, due to the limited therapeutic strategies in inhibition of gastric cancer, further studies are still required to develop effective treatments. In the present study, we attempted to explore the effects of catalpol, extracted from a traditional Chinese herb Rehmannia glutinosa, on gastric cancer progression in cells and in xenograft nude mice. The results indicated that catalpol dose-dependently reduced the proliferation of cancer cells. The migrated cells were also decreased with catalpol treatment, as evidenced by the down-regulated expressions of matrix metalloproteinase-2 (MMP-2), alpha-smooth muscle actin (α-SMA), Ras homolog gene family, member A (RhoA), Rho kinase 1 (ROCK1) and N-cadherin. Further, catalpol induced apoptosis in gastric cancer cells. Apoptosis-related markers including cleaved Caspase-3 and PARP were highly expressed in catalpol-treated cells. However, the cells with pre-treatment of caspases inhibitor reversed catalpol-induced apoptosis. Further, catalpol also enhanced reactive oxygen species (ROS) generation in gastric cancer cells, which was eliminated by N-acetylcystein (NAC) pre-incubation, an important ROS scavenger. Of note, catalpol potentiated the anti-cancer effects of cisplatin (DDP) in suppressing gastric cancer cells. In vivo, catalpol prevented the tumor growth in xenograft nude mice, while no significant difference was observed in body weight. The immunohistochemical analysis showed that catalpol increased the number of terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL)-positive cells, whereas decreased the number of KI-67-positive cells. Together, the results above indicated that catalpol has a potential value in treating gastric cancer.
[Study on quality evaluation of Dihuang (Rehmannia glutinosa) by two-dimension HPLC fingerprints and chemometrics methods].[Pubmed: 29751715]
The study is to establish the two-dimension HPLC fingerprints of Dihuang (Rehmannia glutinosa), by HPLC-PDA and HPLC-ELSD methods. The separations were performed on Waters Atlantis®T3(4.6 mm× 250 mm,5 μm)and Welch Ultimate®Hilic-NH₂(4.6 mm× 250 mm,5 μm)columns with the gradient elution of acetonitrile-0.01% phosphoric acid and acetonitrile-water, respectively. The chromatographic display wavelength for PDA detector was set at 203 nm. For HPLC-ELSD, the nebulizer was set as cooling mode, the drift tube temperature was set at 60 °C and the gas pressure was 35.0 psi. Based on similarity evaluation system for chromatographic fingerprint of traditional Chinese medicine, 26 and 10 chromatographic peaks were determined as common components for HPLC-PDA and HPLC-ELSD fingerprints, respectively. Chemometrics analyses, such as similarity analysis; cluster analysis and principal component analysis, were performed on the common peak areas in two-dimension fingerprints for 41 batches of Dihuang from multiple sources. The results showed that the HPLC-PDA fingerprint could distinguish dried rehmannia root between different sources, and HPLC-ELSD fingerprint could differentiate dried rehmannia root from prepared rehmannia root. The two-dimension fingerprints were established with advantages of a good degree of separation, abundant chemical information and multi-components identified including two nucleosides (adenosine and uridine),four iridoid glycosides (catalpa alcohol,rehmaionoside D,rehmaionoside A and leonuride),two phenylethanoid glycosides (acteoside and cistanoside A) and nine sugars. The method is simple and practical, which could be used for the identification and quality assessment for Dihuang.
[Changes of transport sugar content in different organs of Rehmannia glutinosa].[Pubmed: 29751701]
Raffinose series oligosaccharides are the transport and storage sugars of many plants, Rehmannia glutinosa is one of the commonly used Chinese herbal medicines, medicinal parts ist he roots. Root and tuber of R. glutinosa contains stachyose, raffinose and other oligosaccharides, but the study about the process of growth and development of other organs in the non-structural changes in sugar content is rare.In this study, leaves, stems and roots of R. glutinosa were used as materials to analyze the diurnal variation and the changes of sugar content of sucrose, raffinose and stachyose in different organs of R. glutinosa. The results showed that the content of sucrose in R. glutinosa leaves gradually increased from seedling stage.However, the content of stachyose did not change much at the early stage of growth, and the stachyose rapidly increased at the later stage of growth. The raffinose content gradually decreased throughout the growing season, young leaves of R. glutinosa have higher ability to sucrose synthesis than mature leaves, while mature leaf has higher raffinose and stachyose synthesis ability than young leaves. Sucrose and stachyose content in stem gradually increased, while there was little change in raffinose content. The content of raffinose and stachyose in root increased rapidly from the beginning of fast growing period, while the content of sucrose did not change much. The content of sucrose in leaves of R. glutinosa did not change much at day and night, while the daily changes of raffinose and stachyose contents were very obvious. The contents of raffinose and stachyose in daytime were higher than those at night. The content of raffinose in root and stem was not changed much, but the change of stachyose in root, stem and leaf was very obvious, especially in stem and leaf. In summary, the leaf is the main synthetic organ of raffinose, leaves, stems and roots are stachyose synthesis organ. Sucrose, raffinose and stachyose are the major transport forms of carbohydrates in R. glutinosa.
Rehmannia glutinosa polysaccharide promoted activation of human dendritic cells.[Pubmed: 29715554]
In our previous study, we showed that Rehmannia glutinosa polysaccharide (RGP) treatment induced maturation of dendritic cells (DCs) and that it had an anticancer effect in mice. The effect of RGP has not been studied in human DCs, including monocyte-derived DCs (MDDCs) and peripheral blood DCs (PBDCs). In this study, we examined DC activation by RGP in human cells. The dendritic morphology of RGP-treated MDDCs was substantially altered as compared with that of phosphate-buffered saline (PBS)-treated control cells. Moreover, RGP treatment markedly decreased phagocytic activity and increased expression levels of co-stimulatory molecules in MDDCs. In addition, RGP treatment elevated the production of proinflammatory cytokines. Furthermore, RGP-induced activation of MDDCs was dependent on the phosphorylation of extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK). RGP-treated MDDCs promoted upregulation of T-cell activation, including proliferation and interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α) production. Analysis of the effect of RGP in PBDC subsets revealed that it induced upregulation of co-stimulatory molecule expression and proinflammatory cytokine production. These data suggest that RGP may function as an immune stimulatory molecule in humans.


