Saposhnikovia divaricata
Saposhnikovia divaricata
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
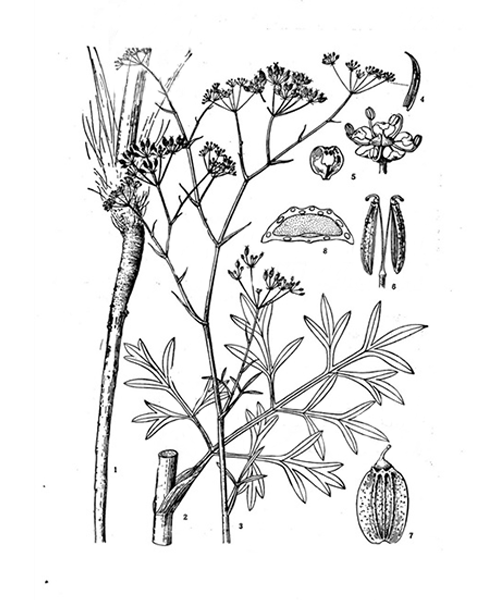
Natural products/compounds from Saposhnikovia divaricata
- Cat.No. Product Name CAS Number COA
-
BCN8288
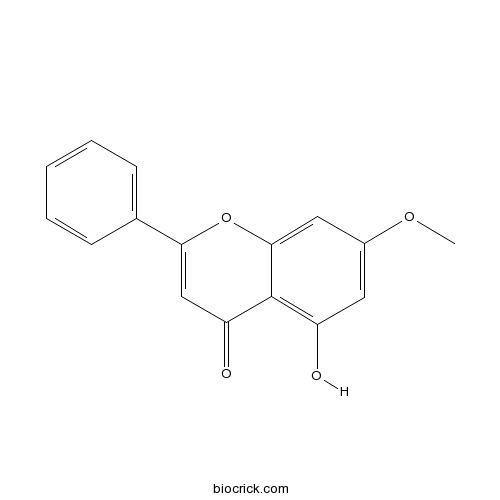
S-(+)-Marmesin13849-08-6
Instructions

-
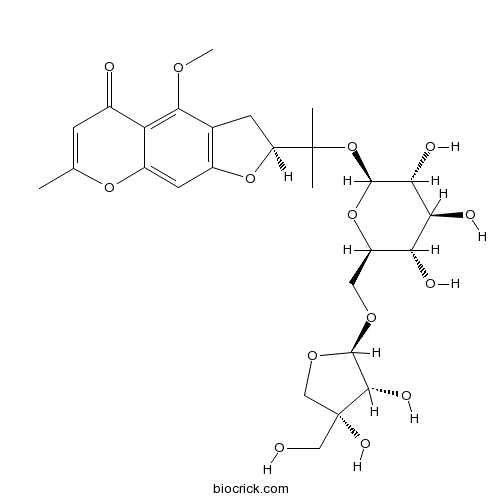
BCN7858
6-O-apiosyl-5-O-Methylvisammioside139446-82-5
Instructions
-
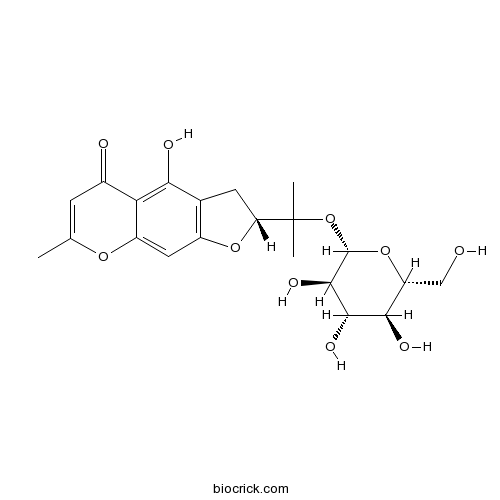
BCN7857
Monnieriside G1401799-34-5
Instructions
-
BCN7853
Cimifugin 4'-O-beta-D-glucopyranoside1632110-81-6
Instructions

-
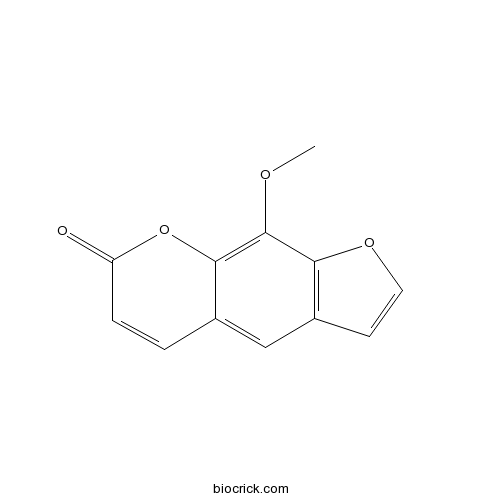
BCN5205
Xanthotoxin298-81-7
Instructions
-
BCN5433
Cimifugin37921-38-3
Instructions

-
BCN5574
Imperatorin482-44-0
Instructions
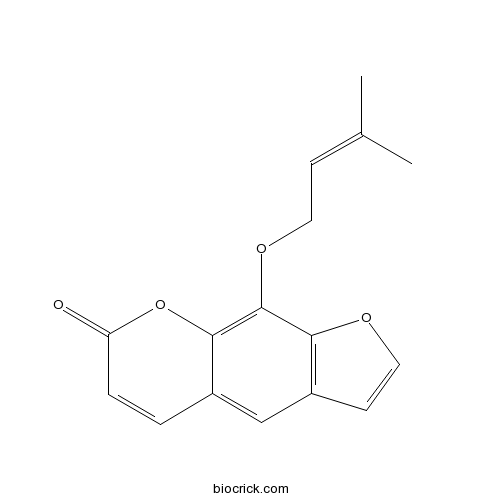
-
BCN2377
Isobergapten482-48-4
Instructions
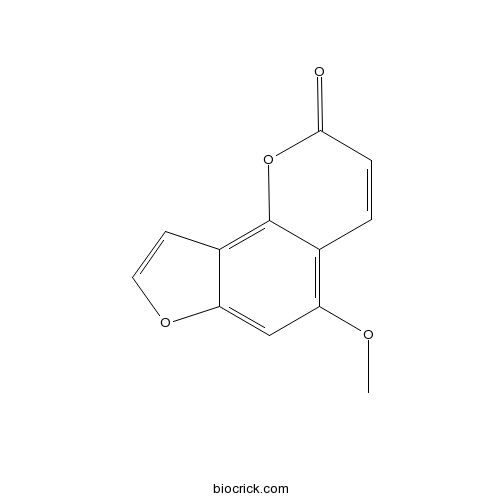
-
BCN5582
Bergapten484-20-8
Instructions
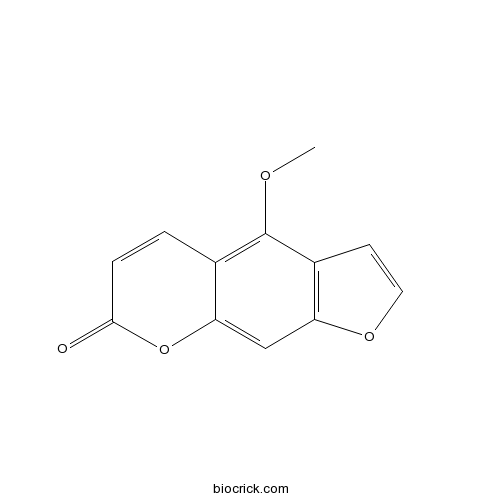
-
BCN5604
Nodakenetin495-32-9
Instructions
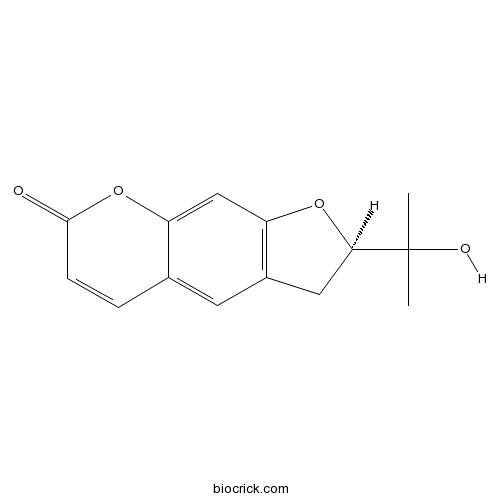
-
BCN5655
Tectochrysin520-28-5
Instructions
-
BCN5335
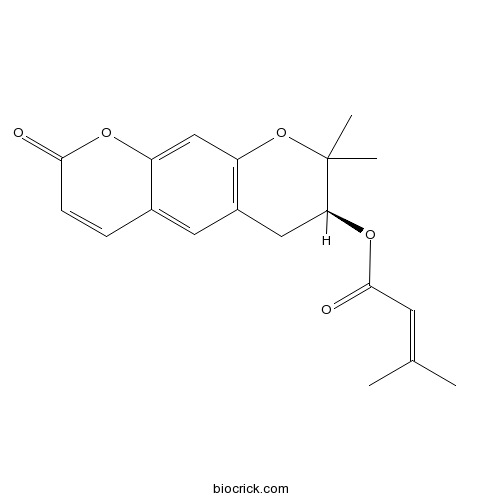
Decursin5928-25-6
Instructions
-
BCN2205
D-Mannitol69-65-8
Instructions

-
BCN1233
Sec-O-Glucosylhamaudol80681-44-3
Instructions

-
BCN4952
Prim-O-glucosylcimifugin80681-45-4
Instructions

-
BCN6309
Coumarin91-64-5
Instructions

Transcriptomic analysis identifies differentially expressed genes (DEGs) associated with bolting and flowering in Saposhnikovia divaricata.[Pubmed: 30047466]
Saposhnikovia divaricata is a valuable Chinese medicinal herb; the transformation from vegetative growth to reproductive growth may lead to the decrease of its pharmacological activities. Therefore, the study of bolting and flowering for Saposhnikovia divaricata is warranted. The present study aimed to reveal differentially expressed genes (DEGs) and regularity of expression during the bolting and flowering process, and the results of this study might provide a theoretical foundation for the suppression of early bolting for future research and practical application. Three sample groups, early flowering, flower bud differentiation, and late flowering (groups A, B, and C, respectively) were selected. Transcriptomic analysis identified 67, 010 annotated unigenes, among which 50, 165 were differentially expressed including 16, 108 in A vs B, and 17, 459 in B vs C, respectively. Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway functional classification analysis were performed on these differentially expressed genes, and five important pathways were significantly impacted (P ≤ 0.01): plant circadian rhythm, other glycan degradation, oxidative phosphorylation, plant hormone signal transduction, and starch and sucrose metabolism. Plant hormone signal transduction might play an important role in the bolting and flowering process. The differentially expressed indole-3-acetic acid (IAA) gene showed significant down-regulation during bolting and flowering, while the transport inhibitor response 1 (TIR1) gene showed no significant change during the bolting process. The expression of flowering related genes FLC, LYF, and AP1 also showed a greater difference at different development stages. In conclusion, we speculate that the decrease in auxin concentration is not caused by the degrading effect of TIR1 but by an alternative mechanism.
Two New Chromone Glycosides from Roots of Saposhnikovia divaricata.[Pubmed: 29987893]
None
Structural characterization of polysaccharides from Saposhnikovia divaricata and their antagonistic effects against the immunosuppression by the culture supernatants of melanoma cells on RAW264.7 macrophages.[Pubmed: 29518443]
None
Ultra-performance convergence chromatography method for the determination of four chromones and quality control of Saposhnikovia divaricata (Turcz.) Schischk.[Pubmed: 29280307]
None
Ameliorative effect of panaxynol on the reduction in high-molecular-weight adiponectin secretion from 3T3-L1 adipocytes treated with palmitic acids.[Pubmed: 29248425]
Reduced plasma levels of the high-molecular weight (HMW) form of adiponectin, rather than total adiponectin levels, have been shown to be closely associated with various metabolic diseases including insulin resistance, type 2 diabetes, and cardiovascular disease. Therefore, we sought to explore active, naturally occurring compounds that promote the recovery of HMW adiponectin secretion suppressed by palmitic acid in our model. A total of 90 crude drug extracts were screened for the ability to augment HMW adiponectin secretion from 3T3-L1 adipocytes treated with palmitic acid. Panaxynol was isolated from Saposhnikovia divaricata as an active compound with HMW adiponectin promoting properties. Peroxisome proliferator-activated receptor-γ (PPARγ) agonists are reported to increase the secretion of HMW adiponectin, although the effects of panaxynol were found to be independent of PPARγ activation. When the underlying mechanisms were further examined, panaxynol was found to inhibit the palmitic-acid-induced downregulation of forkhead box O1 (FoxO1) protein, and the anti-lipotoxic effects were abolished by a FoxO1 inhibitor. Furthermore, CCAAT/enhancer-binding protein-α (C/EBPα) mRNA levels were also increased by panaxynol. Reactive oxygen species have critical roles in the reduction in HMW adiponection secretion by palmitic acid; however, panaxynol reduced this increase in reactive oxygen species generation, followed by reductions in markers of endoplasmic reticulum stress and inflammation. Taken together, these findings suggest that panaxynol ameliorates the impaired HMW adiponection secretion in adipocytes treated with palmitic acid by restoring FoxO1 expression, owing to inhibition of reactive oxygen species generation, in a PPARγ-independent manner.
Oviposition inhibitor in umbelliferous medicinal plants for the common yellow swallowtail (Papilio machaon).[Pubmed: 28884433]
Umbelliferous medicinal plants, such as Angelica acutiloba Kitagawa and Angelica dahurica Bentham et Hooker filius ex Franchet et Savatier, account for a large percentage of crude drug consumption in Japan. The most serious problem in the cultivation of umbelliferous medicinal plants is the feeding damage caused by the common yellow swallowtail (Papilio machaon hippocrates C. & R. Felder, 1864). When we compared the numbers of eggs laid by P. machaon on six umbelliferous medicinal plants, the eggs on A. acutiloba, A. dahurica, and Glehnia littoralis Fr. Schmidt ex Miquel were the most numerous, those on Saposhnikovia divaricata Schischkin and Cnidium officinale Makino were rare, and Bupleurum falcatum Linné was not oviposited at all. To identify oviposition inhibitors for P. machaon in B. falcatum, S. divaricata, and C. officinale, the volatile chemical constituents of these umbelliferous medicinal plants were compared with GC-MS. We carried out multivariate analysis of gas chromatographic data and concluded that germacrene D, α-humulene, and trans-caryophyllene play important roles in protecting plants from oviposition by P. machaon. Their oviposition repellent activity was confirmed by the fact that the number of eggs laid on the leaves around a repellent device containing a mixture of germacrene D, α-humulene, and trans-caryophyllene was reduced by 40% compared to a control.
Rapid characterisation and identification of compounds in Saposhnikoviae Radix by high-performance liquid chromatography coupled with electrospray ionisation quadrupole time-of-flight mass spectrometry.[Pubmed: 28817985]
Saposhnikoviae Radix (SR), the dried root of Saposhnikovia divaricata (Turcz.) Schischk. (Umbelliferae), is commonly used as a traditional Chinese medicine. In this study, a rapid and accurate method was firstly, developed for the qualitative analysis of SR by high-performance liquid chromatography coupled with electrospray ionisation quadrupole time-of-flight mass spectrometry (HPLC-ESI-Q-TOF-MS/MS). A total of 45 compounds were identified or tentatively characterised, including 13 chromones, 28 coumarins and four others. Among them, 16 compounds were identified from SR for the first time. In addition, six chromones reference standards, including two isolated compounds of 3'-O-angeloylhamaudol and norcimifugin from the extraction of SR, were used to study the fragmentation pathways of chromones. The developed method was effective for characterising the compounds of SR, and the results of the study enriched the understanding of the chemical connotation.


