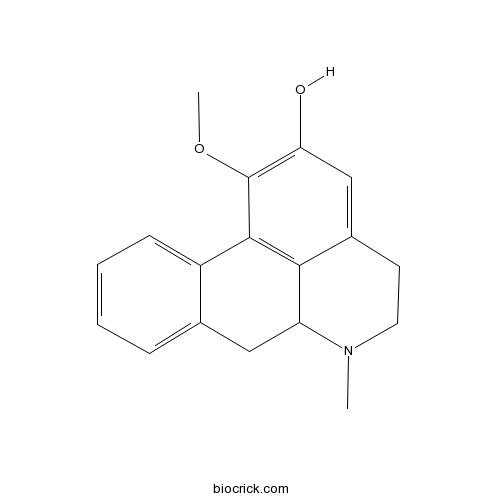
2-Hydroxy-1-MethoxyaporphineCAS# 33770-27-3 |
- O-Nornuciferine
Catalog No.:BCN7074
CAS No.:3153-55-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 33770-27-3 | SDF | Download SDF |
| PubChem ID | 5319512 | Appearance | Powder |
| Formula | C18H19NO2 | M.Wt | 281.35 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Floribundine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 1-methoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinolin-2-ol | ||
| SMILES | CN1CCC2=CC(=C(C3=C2C1CC4=CC=CC=C43)OC)O | ||
| Standard InChIKey | AKXOIHNFHOEPHN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H19NO2/c1-19-8-7-12-10-15(20)18(21-2)17-13-6-4-3-5-11(13)9-14(19)16(12)17/h3-6,10,14,20H,7-9H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 2-Hydroxy-1-Methoxyaporphine can inhibit CYP2D6 activity, it also increase the glucose consumption significantly as rosiglitazone. |
| Targets | CYP2D6 |
| In vitro | Purification and characterization of aporphine alkaloids from leaves of Nelumbo nucifera Gaertn and their effects on glucose consumption in 3T3-L1 adipocytes.[Pubmed: 24577311 ]Int J Mol Sci. 2014 Feb 26;15(3):3481-94.Aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn are substances of great interest because of their important pharmacological activities, particularly anti-diabetic, anti-obesity, anti-hyperlipidemic, anti-oxidant, and anti-HIV's activities.
|
| Kinase Assay | Identification and characterization of potent CYP2D6 inhibitors in lotus leaves.[Pubmed: 24561383 ]J Ethnopharmacol. 2014 Apr 11;153(1):190-6.The herb of lotus (Nelumbo nucifera) leaves is a commonly used traditional Chinese herbal medicine that is utilized for the treatment of sunstroke, to assuage thirst, and to cure both diarrhea and fever in China. Modern pharmacological studies have demonstrated that the herb exhibits various pharmacological effects, such as anti-hyperlipidemia, anti-obesity, anti-oxidant, anti-HIV, anti-microbial, and anti-hypoglycemic activities. Currently, the herb is becoming more popular in China as a "tea drink" or as a main ingredient of some herbal formulations, which implies that the herb and/or its products are now more likely to be concurrently administered with conventional medicines for losing body weight and reducing blood lipids. However, its potential inhibitory effect on human cytochrome P450 (CYP) has not been systemically investigated to date. The present study was performed to assess the potential inhibitory effects of lotus leaf alcoholic extract (LAE), its major fractions, and its main compounds on five CYP isoenzymes (CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) in vitro.
|

2-Hydroxy-1-Methoxyaporphine Dilution Calculator

2-Hydroxy-1-Methoxyaporphine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.5543 mL | 17.7715 mL | 35.5429 mL | 71.0858 mL | 88.8573 mL |
| 5 mM | 0.7109 mL | 3.5543 mL | 7.1086 mL | 14.2172 mL | 17.7715 mL |
| 10 mM | 0.3554 mL | 1.7771 mL | 3.5543 mL | 7.1086 mL | 8.8857 mL |
| 50 mM | 0.0711 mL | 0.3554 mL | 0.7109 mL | 1.4217 mL | 1.7771 mL |
| 100 mM | 0.0355 mL | 0.1777 mL | 0.3554 mL | 0.7109 mL | 0.8886 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7,22,25-Stigmastatrienol
Catalog No.:BCN8816
CAS No.:14485-48-4
- Chrysoeriol 7-apiosylglucoside
Catalog No.:BCN8815
CAS No.:33579-63-4
- 2,4,7-Trihydroxy-9,10-dihydrophenanthrene
Catalog No.:BCN8814
CAS No.:70205-52-6
- Rhaponticin 2''-O-gallate
Catalog No.:BCN8813
CAS No.:94356-24-8
- 6-Hydroxy-2-(2-phenylethyl)chromone
Catalog No.:BCN8812
CAS No.:84294-90-6
- 6,7-Dimethoxy-2-phenethylchromone
Catalog No.:BCN8811
CAS No.:84294-87-1
- 6-Hydroxyluteolin 7-glucoside
Catalog No.:BCN8810
CAS No.:54300-65-1
- Volvalerenic acid A
Catalog No.:BCN8809
CAS No.:1247014-34-1
- Heishuixiecaoline A
Catalog No.:BCN8808
CAS No.:1469493-85-3
- Kalopanaxsaponin G
Catalog No.:BCN8806
CAS No.:171370-50-6
- Sterebin A
Catalog No.:BCN8802
CAS No.:107647-14-3
- Isoagarotetrol
Catalog No.:BCN8801
CAS No.:104060-61-9
- Aloe-emodin-3-(hydroxymethyl)-O-beta-D-glucopyranoside
Catalog No.:BCN8818
CAS No.:50488-89-6
- 5,7,3',4'-Tetramethoxyflavone
Catalog No.:BCN8821
CAS No.:855-97-0
- Combretol
Catalog No.:BCN8822
CAS No.:5084-19-5
- Ardisiacrispin B
Catalog No.:BCN8823
CAS No.:112766-96-8
- Ardisicrenoside A
Catalog No.:BCN8824
CAS No.:160824-52-2
- Chrysophanol 1-O-beta-tetraglucoside
Catalog No.:BCN8825
CAS No.:120181-08-0
- 11-Oxomogroside IV
Catalog No.:BCN8826
CAS No.:2096516-32-2
- 5'''-O-Feruloyl complanatoside B
Catalog No.:BCN8827
CAS No.:142473-98-1
- Isorhamnetin 3,7-O-diglucoside
Catalog No.:BCN8828
CAS No.:6758-51-6
- Anemarrhenasaponin III
Catalog No.:BCN8829
CAS No.:163047-23-2
- Cassiaside B2
Catalog No.:BCN8830
CAS No.:218155-40-9
- Harmidol hydrochloride
Catalog No.:BCN8831
CAS No.:6028-07-5
Purification and characterization of aporphine alkaloids from leaves of Nelumbo nucifera Gaertn and their effects on glucose consumption in 3T3-L1 adipocytes.[Pubmed:24577311]
Int J Mol Sci. 2014 Feb 26;15(3):3481-94.
Aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn are substances of great interest because of their important pharmacological activities, particularly anti-diabetic, anti-obesity, anti-hyperlipidemic, anti-oxidant, and anti-HIV's activities. In order to produce large amounts of pure alkaloid for research purposes, a novel method using high-speed counter-current chromatography (HSCCC) was developed. Without any initial cleanup steps, four main aporphine alkaloids, including 2-Hydroxy-1-Methoxyaporphine, pronuciferine, nuciferine and roemerine were successfully purified from the crude extract by HSCCC in one step. The separation was performed with a simple two-phase solvent system composed of n-hexane-ethyl acetate-methanol-acetonitrile-water (5:3:3:2.5:5, v/v/v/v/v). In each operation, 100 mg crude extracts was separated and yielded 6.3 mg of 2-Hydroxy-1-Methoxyaporphine (95.1% purity), 1.1 mg of pronuciferine (96.8% purity), 8.5 mg of nuciferine (98.9% purity), and 2.7 mg of roemerine (97.4%) respectively. The chemical structure of four aporphine alkaloids are identified by means of electrospray ionization MS (ESI-MS) and nuclear magnetic resonance (NMR) analysis. Moreover, the effects of four separated aporphine alkaloids on insulin-stimulated glucose consumption were examined in 3T3-L1 adipocytes. The results showed that 2-Hydroxy-1-Methoxyaporphine and pronuciferine increased the glucose consumption significantly as rosiglitazone did.
Identification and characterization of potent CYP2D6 inhibitors in lotus leaves.[Pubmed:24561383]
J Ethnopharmacol. 2014 Apr 11;153(1):190-6.
ETHNOPHARMACOLOGICAL RELEVANCE: The herb of lotus (Nelumbo nucifera) leaves is a commonly used traditional Chinese herbal medicine that is utilized for the treatment of sunstroke, to assuage thirst, and to cure both diarrhea and fever in China. Modern pharmacological studies have demonstrated that the herb exhibits various pharmacological effects, such as anti-hyperlipidemia, anti-obesity, anti-oxidant, anti-HIV, anti-microbial, and anti-hypoglycemic activities. Currently, the herb is becoming more popular in China as a "tea drink" or as a main ingredient of some herbal formulations, which implies that the herb and/or its products are now more likely to be concurrently administered with conventional medicines for losing body weight and reducing blood lipids. However, its potential inhibitory effect on human cytochrome P450 (CYP) has not been systemically investigated to date. The present study was performed to assess the potential inhibitory effects of lotus leaf alcoholic extract (LAE), its major fractions, and its main compounds on five CYP isoenzymes (CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) in vitro. MATERIAL AND METHODS: Five probe substrates were incubated with human liver microsomes in the presence or absence of the LAE, the alkaloid fraction (AF), the flavonoid fraction (FF), or the individual aporphine alkaloids, namely, nuciferine (NF), N-nornuciferine (N-NF), and 2-Hydroxy-1-Methoxyaporphine (HMA). After the incubation, the relative metabolites of the substrates were analyzed using LC-MS/MS. RESULTS: The results showed that the LAE strongly inhibited CYP2D6 with an IC50 value of 12.05microg/mL and weakly inhibited other isoenzymes. In addition, FF was found to weakly inhibit CYP2D6, whereas AF exerted a markedly higher inhibitory effect on CYP2D6 activity with an IC50 value of 0.96microg/mL. The three aporphine alkaloids isolated from the AF (NF, N-NF, and HMA) significantly inhibited CYP2D6 with IC50 values of 3.78, 3.76, and 3.15microM, respectively. Their Lineweaver-Burk plots and Dixon plots showed that NF, N-NF, and HMA competitively inhibited CYP2D6 activity with Ki values of 1.88, 2.34, and 1.56microM, respectively. CONCLUSION: The study revealed that the alkaloid compounds in lotus leaves exert a potent inhibitory effect on CYP2D6 isoenzyme. The possible drug interactions of the leaves and their preparations with conventional medicines should thus be taken into account.
[HPLC determination of 2-hydroxy-1-methoxyaporphine, pronuciferine, nuciferine and roemerine in Nelumbo nucifera and its alkaloid fraction].[Pubmed:18841775]
Zhongguo Zhong Yao Za Zhi. 2008 Jul;33(14):1713-6.
OBJECTIVE: To establish an HPLC method for the determination of four alkaloids, i.e., 2-Hydroxy-1-Methoxyaporphine, pronuciferine, nuciferine and roemerine, in Nelumbo nucifera and its alkaloid fraction. METHOD: The determination was carried out at 35 degrees C on a Hypersil C18 column (4.6 mm x 250 mm, 5 microm), eluting with acetonitrile-water containing 0.1% triethylamine as mobile phases in gradient mode. The flow rate was 1.0 mL x min(-1) and detection at the wavelength was set at 270 nm. RESULT: The linear ranges of 2-Hydroxy-1-Methoxyaporphine, pronuciferine, nuciferine and roemerine were 0.110-0.658 microg (r = 0.9995), 0.0210-0.126 microg (r = 0.9995), 0.103-0.618 microg (r = 0.9998), 0.085 6-0.514 microg (r = 0.9995), with the average recoveries (n=6) were 101.5%, 99.14%, 99.21% and 98.41% for the alkaloid fraction of N. nucifera and 99.53%, 100.5%, 97.51% and 100.1% for N. nucifera respectively. CONCLUSION: The determination results of the three batches of samples showed that the method was easy and accurate which could be used to determine the contents of four components in N. nucifera and its alkaloid fraction.


