CombretolCAS# 5084-19-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 5084-19-5 | SDF | Download SDF |
| PubChem ID | 12303802 | Appearance | Powder |
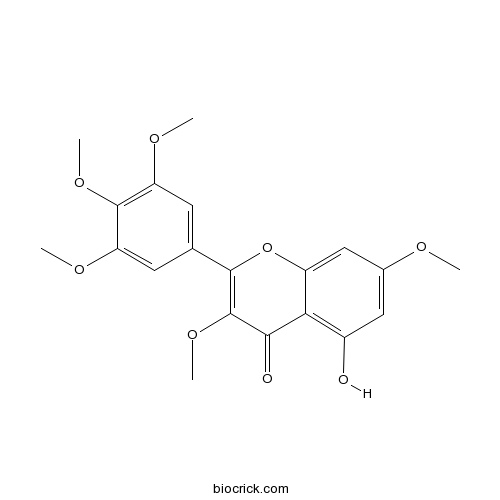
| Formula | C20H20O8 | M.Wt | 388.4 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | 5-Hydroxy-3,3',4',5',7-pentamethoxyflavone | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 5-hydroxy-3,7-dimethoxy-2-(3,4,5-trimethoxyphenyl)chromen-4-one | ||
| SMILES | COC1=CC(=C2C(=C1)OC(=C(C2=O)OC)C3=CC(=C(C(=C3)OC)OC)OC)O | ||
| Standard InChIKey | SUNUQCQIFHHEOW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20O8/c1-23-11-8-12(21)16-13(9-11)28-18(20(27-5)17(16)22)10-6-14(24-2)19(26-4)15(7-10)25-3/h6-9,21H,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Combretol has leishmanicidal activity, it showed moderate activity (growth inhibition 87.3 and 73.0 %, respectively, at 50 µM) against a multidrug-resistant L. tropica line. Combretol also shows weakly active cytotoxic activity. |
| Targets | Antifection |
| In vitro | Leishmanicidal and reversal multidrug resistance constituents from Aeonium lindleyi.[Pubmed: 20665372 ]Planta Med. 2011 Jan;77(1):77-80.
Cytotoxic diterpenes from Cassipourea madagascariensis from the Madagascar rainforest.[Pubmed: 16499334 ]J Nat Prod. 2006 Feb;69(2):287-9.
|

Combretol Dilution Calculator

Combretol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5747 mL | 12.8733 mL | 25.7467 mL | 51.4933 mL | 64.3666 mL |
| 5 mM | 0.5149 mL | 2.5747 mL | 5.1493 mL | 10.2987 mL | 12.8733 mL |
| 10 mM | 0.2575 mL | 1.2873 mL | 2.5747 mL | 5.1493 mL | 6.4367 mL |
| 50 mM | 0.0515 mL | 0.2575 mL | 0.5149 mL | 1.0299 mL | 1.2873 mL |
| 100 mM | 0.0257 mL | 0.1287 mL | 0.2575 mL | 0.5149 mL | 0.6437 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7,3',4'-Tetramethoxyflavone
Catalog No.:BCN8821
CAS No.:855-97-0
- Aloe-emodin-3-(hydroxymethyl)-O-beta-D-glucopyranoside
Catalog No.:BCN8818
CAS No.:50488-89-6
- 2-Hydroxy-1-Methoxyaporphine
Catalog No.:BCN8817
CAS No.:33770-27-3
- 7,22,25-Stigmastatrienol
Catalog No.:BCN8816
CAS No.:14485-48-4
- Chrysoeriol 7-apiosylglucoside
Catalog No.:BCN8815
CAS No.:33579-63-4
- 2,4,7-Trihydroxy-9,10-dihydrophenanthrene
Catalog No.:BCN8814
CAS No.:70205-52-6
- Rhaponticin 2''-O-gallate
Catalog No.:BCN8813
CAS No.:94356-24-8
- 6-Hydroxy-2-(2-phenylethyl)chromone
Catalog No.:BCN8812
CAS No.:84294-90-6
- 6,7-Dimethoxy-2-phenethylchromone
Catalog No.:BCN8811
CAS No.:84294-87-1
- 6-Hydroxyluteolin 7-glucoside
Catalog No.:BCN8810
CAS No.:54300-65-1
- Volvalerenic acid A
Catalog No.:BCN8809
CAS No.:1247014-34-1
- Heishuixiecaoline A
Catalog No.:BCN8808
CAS No.:1469493-85-3
- Ardisiacrispin B
Catalog No.:BCN8823
CAS No.:112766-96-8
- Ardisicrenoside A
Catalog No.:BCN8824
CAS No.:160824-52-2
- Chrysophanol 1-O-beta-tetraglucoside
Catalog No.:BCN8825
CAS No.:120181-08-0
- 11-Oxomogroside IV
Catalog No.:BCN8826
CAS No.:2096516-32-2
- 5'''-O-Feruloyl complanatoside B
Catalog No.:BCN8827
CAS No.:142473-98-1
- Isorhamnetin 3,7-O-diglucoside
Catalog No.:BCN8828
CAS No.:6758-51-6
- Anemarrhenasaponin III
Catalog No.:BCN8829
CAS No.:163047-23-2
- Cassiaside B2
Catalog No.:BCN8830
CAS No.:218155-40-9
- Harmidol hydrochloride
Catalog No.:BCN8831
CAS No.:6028-07-5
- Randialic acid B
Catalog No.:BCN8832
CAS No.:14021-14-8
- Hythiemoside B
Catalog No.:BCN8833
CAS No.:853267-90-0
- Notoginsenoside R4
Catalog No.:BCN8834
CAS No.:87741-77-3
Five new diarylpropan-1-ols from Combretum yunnanense.[Pubmed:21674439]
Planta Med. 2011 Nov;77(16):1841-4.
Five new 1,3-diarylpropan-1-ols, Combretol A-E (1- 5), together with one known coumarin (6) and ten known triterpenes (7-16), were isolated from Combretum yunnanense. Their structures were determined by spectroscopic investigation, including (1)H and (1)(3)C NMR, NOESY, HSQC, HMBC, and HRESIMS analyses. This is the first report on the occurrence of 1,3-diarylpropan-1-ols and coumarin in the Combretum genus including C. yunnanense. Also, to the best of our knowledge, 1,3-diarylpropan-1-ols are rare in nature.
Leishmanicidal and reversal multidrug resistance constituents from Aeonium lindleyi.[Pubmed:20665372]
Planta Med. 2011 Jan;77(1):77-80.
A new bicyclic diterpene with a labdane skeleton, 7-oxo-labd-8-en-15-ol ( 1), along with two known diterpenes and ten flavonoids were isolated from the leaves of Aeonium lindleyi (Crassulaceae). Their structures were elucidated on the basis of spectroscopic data, including 1D and 2D NMR experiments, and comparison with spectroscopic data reported in the literature. Labdan-8 alpha,15-diol (2) and labd-8(17)-en-3 beta,15-diol (3) showed leishmanicidal activity against Leishmania tropica (IC (50) = 77.0 microM) and Leishmania braziliensis (IC (50) = 68.0 microM) similar to ketoconazole used as positive control. 5,3'-Dihydroxy-3,7,4',5'-tetramethoxyflavone (8) and Combretol (9) showed moderate activity (growth inhibition 87.3 and 73.0 %, respectively, at 50 microM) against a multidrug-resistant L. tropica line.
Cytotoxic diterpenes from Cassipourea madagascariensis from the Madagascar rainforest.[Pubmed:16499334]
J Nat Prod. 2006 Feb;69(2):287-9.
Bioassay-directed fractionation of ethanol extracts of the roots and leaves of the plant Cassipourea madagascariensis resulted in the isolation of the two new terpenoids cassipourol (1) and cassipouryl acetate (2) in addition to the three known compounds, 3beta,30-dihydroxylup-20(29)-ene (3), 30-hydroxylup-20(29)-en-3-one (4), and Combretol (5). The structures of the two new compounds were established on the basis of 1D and 2D NMR spectroscopic data and chemical conversion. All the isolated compounds were tested against the A2780 human ovarian cancer cell line; the two diterpenes (1 and 2) showed moderate cytotoxic activity, while the three known compounds (3-5) were weakly active.


