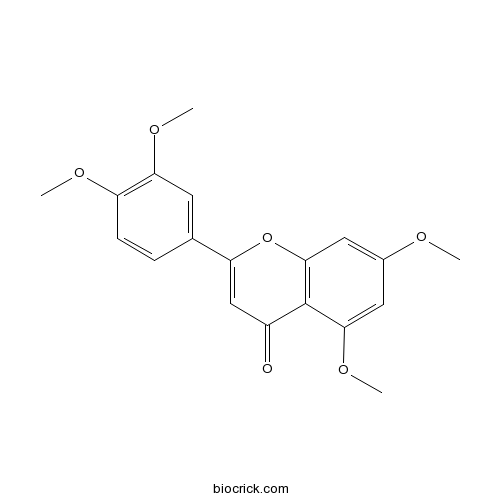
5,7,3',4'-TetramethoxyflavoneCAS# 855-97-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 855-97-0 | SDF | Download SDF |
| PubChem ID | 631170 | Appearance | Powder |
| Formula | C19H18O6 | M.Wt | 342.3 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | DMSO : 8.33 mg/mL (24.33 mM; Need ultrasonic) | ||
| Chemical Name | 2-(3,4-dimethoxyphenyl)-5,7-dimethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(O2)C=C(C=C3OC)OC)OC | ||
| Standard InChIKey | CLXVBVLQKLQNRQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H18O6/c1-21-12-8-17(24-4)19-13(20)10-15(25-18(19)9-12)11-5-6-14(22-2)16(7-11)23-3/h5-10H,1-4H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 5,7,3',4'-Tetramethoxyflavone (TMF) possesses various bioactivities, including antifungal, antimalarial, antimycobacterial, and anti-inflammatory activities; it also exhibits chondroprotective activity by targeting β-catenin signaling in vivo and in vitro. TMF protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1α pathway.TMF inhibits the expression of tyrosinase, tyrosine-related protein (TRP)-1, and TRP-2 mRNA, which could be the mechanism of its melanogenesis inhibitory activity. |
| Targets | β-catenin | IRE1α | TRP-2 | TRP-1 | Antifection | TNF-α | PGE | PKA | cAMP | JNK | Caspase | Bcl/Bax | Antifection |
| In vitro | 5,7,3',4'-Tetramethoxyflavone protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1α pathway.[Pubmed: 28436754 ]Connect Tissue Res. 2018 Mar;59(2):157-166.To investigate the roles of endoplasmic reticulum (ER) transmembrane sensor inositol-requiring enzyme-1 (IRE1)α signaling in ER stress-induced chondrocyte apoptosis, and to determine the molecular mechanisms underlying chondroprotective activity of 5,7,3',4'-Tetramethoxyflavone (TMF) from Murraya exotica.
Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora.[Pubmed: 26711832 ]J Nat Med. 2016 Apr;70(2):179-89.A methanol extract from the rhizomes of Kaempferia parviflora Wall. ex Baker (Zingiberaceae) has shown inhibitory effects against melanogenesis in theophylline-stimulated murine B16 melanoma 4A5 cells (IC50 = 9.6 μg/mL).
|
| In vivo | Identification of 5,7,3',4'-tetramethoxyflavone metabolites in rat urine by the isotope-labeling method and ultrahigh-performance liquid chromatography-electrospray ionization-mass spectrometry.[Pubmed: 22812915 ]J Agric Food Chem. 2012 Aug 22;60(33):8123-8.5,7,3',4'-Tetramethoxyflavone (TMF), one of the major polymethoxyflavones (PMFs) isolated from Kaempferia parviflor , has been reported possessing various bioactivities, including antifungal, antimalarial, antimycobacterial, and anti-inflammatory activities. Although several studies on the TMF have been reported, the information about the metabolism of TMF and the structures of TMF metabolites is still not yet clear.
|
| Kinase Assay | 5,7,3',4'-Tetramethoxyflavone exhibits chondroprotective activity by targeting β-catenin signaling in vivo and in vitro.[Pubmed: 28436754 ]Connect Tissue Res. 2018 Mar;59(2):157-166.Osteoarthritis (OA) is a progressive joint disorder, which remains the leading cause of chronic disability in aged people.
|

5,7,3',4'-Tetramethoxyflavone Dilution Calculator

5,7,3',4'-Tetramethoxyflavone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9214 mL | 14.6071 mL | 29.2141 mL | 58.4283 mL | 73.0353 mL |
| 5 mM | 0.5843 mL | 2.9214 mL | 5.8428 mL | 11.6857 mL | 14.6071 mL |
| 10 mM | 0.2921 mL | 1.4607 mL | 2.9214 mL | 5.8428 mL | 7.3035 mL |
| 50 mM | 0.0584 mL | 0.2921 mL | 0.5843 mL | 1.1686 mL | 1.4607 mL |
| 100 mM | 0.0292 mL | 0.1461 mL | 0.2921 mL | 0.5843 mL | 0.7304 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Aloe-emodin-3-(hydroxymethyl)-O-beta-D-glucopyranoside
Catalog No.:BCN8818
CAS No.:50488-89-6
- 2-Hydroxy-1-Methoxyaporphine
Catalog No.:BCN8817
CAS No.:33770-27-3
- 7,22,25-Stigmastatrienol
Catalog No.:BCN8816
CAS No.:14485-48-4
- Chrysoeriol 7-apiosylglucoside
Catalog No.:BCN8815
CAS No.:33579-63-4
- 2,4,7-Trihydroxy-9,10-dihydrophenanthrene
Catalog No.:BCN8814
CAS No.:70205-52-6
- Rhaponticin 2''-O-gallate
Catalog No.:BCN8813
CAS No.:94356-24-8
- 6-Hydroxy-2-(2-phenylethyl)chromone
Catalog No.:BCN8812
CAS No.:84294-90-6
- 6,7-Dimethoxy-2-phenethylchromone
Catalog No.:BCN8811
CAS No.:84294-87-1
- 6-Hydroxyluteolin 7-glucoside
Catalog No.:BCN8810
CAS No.:54300-65-1
- Volvalerenic acid A
Catalog No.:BCN8809
CAS No.:1247014-34-1
- Heishuixiecaoline A
Catalog No.:BCN8808
CAS No.:1469493-85-3
- Kalopanaxsaponin G
Catalog No.:BCN8806
CAS No.:171370-50-6
- Combretol
Catalog No.:BCN8822
CAS No.:5084-19-5
- Ardisiacrispin B
Catalog No.:BCN8823
CAS No.:112766-96-8
- Ardisicrenoside A
Catalog No.:BCN8824
CAS No.:160824-52-2
- Chrysophanol 1-O-beta-tetraglucoside
Catalog No.:BCN8825
CAS No.:120181-08-0
- 11-Oxomogroside IV
Catalog No.:BCN8826
CAS No.:2096516-32-2
- 5'''-O-Feruloyl complanatoside B
Catalog No.:BCN8827
CAS No.:142473-98-1
- Isorhamnetin 3,7-O-diglucoside
Catalog No.:BCN8828
CAS No.:6758-51-6
- Anemarrhenasaponin III
Catalog No.:BCN8829
CAS No.:163047-23-2
- Cassiaside B2
Catalog No.:BCN8830
CAS No.:218155-40-9
- Harmidol hydrochloride
Catalog No.:BCN8831
CAS No.:6028-07-5
- Randialic acid B
Catalog No.:BCN8832
CAS No.:14021-14-8
- Hythiemoside B
Catalog No.:BCN8833
CAS No.:853267-90-0
TMF inhibits miR-29a/Wnt/beta-catenin signaling through upregulating Foxo3a activity in osteoarthritis chondrocytes.[Pubmed:31354246]
Drug Des Devel Ther. 2019 Jun 19;13:2009-2019.
Background: miR-29a, a downstream factor of Wnt/beta-catenin signaling, promotes the activity of the Wnt/beta-catenin signaling in a positive feedback loop. Our previous work showed that 5,7,3',4'-tetramethoxyflavone (TMF), a major constituent from Murraya exotica L., exhibited chondroprotective activity by inhibiting the activity of Wnt/beta-catenin signaling. Purpose: To investigate whether TMF showed the inhibitory effects on miR-29a/beta-catenin signaling by up regulation of Foxo3a expression. Methods: Rat knee OA models were duplicated by using Hulth's method. TMF (5 mug/mL and 20 mug/mL) was used for administration to cultured cells, which were isolated from the rat cartilages. Analysis of chondrocytes apoptosis, gene expression, and protein expression were conducted. In addition, miR-29a mimics and pcDNA3.1(+)-Foxo3a vector were used for transfection, luciferase reporter assay for detecting the activity of Wnt/beta-catenin signaling, and co-immunoprecipitation for determining proteins interaction. Results: TMF down regulated miR-29a/beta-catenin signaling activity and cleaved caspase-3 expression and up regulated Foxo3a expression in OA rat cartilages. In vitro, miR-29a mimics down regulated the expression of Foxo3a and up regulated the activity of Wnt/beta-catenin signaling and cleaved caspase-3 expression. TMF ameliorated miR-29a/beta-catenin-induced chondrocytes apoptosis by up regulation of Foxo3a expression. Conclusion: TMF exhibited chondroprotective activity by up regulating Foxo3a expression and subsequently inhibiting miR-29a/Wnt/beta-catenin signaling activity.
Establishment and Use of Human Mouth Epidermal Carcinoma (KB) Cells Overexpressing P-Glycoprotein To Characterize Structure Requirements for Flavonoids Transported by the Efflux Transporter.[Pubmed:30688455]
J Agric Food Chem. 2019 Feb 27;67(8):2350-2360.
This study was aimed to determine the mechanism for flavonoid poor absorption related to P-glycoprotein (P-gp). The cellular uptake (CU) of 40 flavonoids was investigated in P-gp overexpressing KB/multidrug-resistant (MDR) cells. A total of 9 flavonoids, including 5,7,3',4'-tetramethoxyflavone, with a significant ( p < 0.05) CUKBE (2.90 +/- 0.146 mumol/g) higher than CUKBP (1.57 +/- 0.129 mumol/g) were identified as P-gp substrates. Besides, 8 substrates, including tangeretin, showed a significant ( p < 0.05) CUKB (9.72 +/- 1.09 mumol/g) higher than its CUKBP (7.36 +/- 0.692 mumol/g). A total of 7 of 17 flavonoid substrates stimulated the P-gp efflux of rhodamine 123, and most substrates increased P-gp expression in KB/MDR cells. Docking analyses showed a good correlation ( R = 0.764; p < 0.01) between efflux fold and S_scoring of flavonoids to the P-gp model, indicating consistency between in silico and in vitro results. A structure-affinity relationship exhibited that 3-OH, 5-OH, 3'-OCH3, and 4'-OCH3 are crucial for flavonoids binding to P-gp. These results provide valuable information for finding a solution to improve the absorption of flavonoids.
SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors.[Pubmed:28461492]
Proc Natl Acad Sci U S A. 2017 May 16;114(20):E4002-E4009.
The peptide substance P (SP) and the cytokine tumor necrosis factor (TNF) have been implicated in inflammatory processes. Mast cells are recognized as important in inflammatory responses. Here, we report that IL-33 (30 ng/mL), a member of the IL-1 family of cytokines, administered in combination with SP (1 microM), markedly increase (by 1,000-fold) TNF gene expression in cultured human LAD2 and primary mast cells derived from umbilical cord blood. SP (0.01-1 muM) and IL-33 (1-100 ng/mL) in combination also greatly stimulate TNF secretion (by 4,500-fold). Pretreatment of LAD2 cells with two different neurokinin-1 (NK-1) receptor antagonists and siRNA inhibits TNF secretion by 50% (P < 0.001) when stimulated by SP and IL-33. Pretreatment of LAD2 cells with a neutralizing antibody for IL-33 receptor, ST2, inhibits TNF secretion by 50% (P < 0.001), and ST2 siRNA decreases TNF secretion by 30% (P < 0.05), when stimulated by SP and IL-33. Surprisingly, NK-1 antagonists also inhibit 50% of TNF secretion (P < 0.001) when stimulated only by IL-33, and ST2 receptor reduction also decreases SP-stimulated TNF secretion by 30% (P < 0.05), suggesting an interaction between NK-1 and ST2 receptors. Moreover, IL-33 increases NK-1 gene and surface protein expression, as well as IKbeta-alpha phosphorylation. Pretreatment of LAD2 cells with 5,7,3',4'-tetramethoxyflavone (methoxyluteolin) (1-100 muM) inhibits (P < 0.001) TNF gene expression (98%) and secretion (64%) at 50 microM and phosphorylation of p-IKB-alpha at 1 muM when stimulated by SP and IL-33. These findings identify a unique amplification process of TNF synthesis and secretion via the interaction of NK-1 and ST2 receptors inhibitable by methoxyluteolin.
5,7,3',4'-Tetramethoxyflavone protects chondrocytes from ER stress-induced apoptosis through regulation of the IRE1alpha pathway.[Pubmed:28436754]
Connect Tissue Res. 2018 Mar;59(2):157-166.
AIM OF THE STUDY: To investigate the roles of endoplasmic reticulum (ER) transmembrane sensor inositol-requiring enzyme-1 (IRE1)alpha signaling in ER stress-induced chondrocyte apoptosis, and to determine the molecular mechanisms underlying chondroprotective activity of 5,7,3',4'-tetramethoxyflavone (TMF) from Murraya exotica. MATERIALS AND METHODS: IRE1alpha was knocked down by siRNA transfection in chondrocytes, which were harvested from rats' knee cartilages. Chondrocytes with IRE1alpha deficiency were administrated with tunicamycin (TM) and TMF. Chondrocyte apoptosis was quantified by flow cytometry and DAPI/TUNEL staining. Expression of mRNA and proteins was quantified by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and western-blot, respectively. RESULTS: IRE1alpha deficiency significantly increased the rate of TM-induced chondrocyte apoptosis, down-regulated the expression of pro-survival factors XBP1S and Bcl-2, and up-regulated pro-apoptotic factors CHOP, p-JNK, and caspase-3. TMF suppressed TM-induced chondrocyte apoptosis by activating the expression of IRE1alpha, which reversed the expression patterns of downstream pro-survival and pro-apoptotic factors due to IRE1alpha deficiency. CONCLUSION: The mechanism of TMF in protecting chondrocytes against ER stress-induced apoptosis might be associated with regulating the activity of ER sensor IRE1alpha and its downstream pathway.
TMF protects chondrocytes from ER stress-induced apoptosis by down-regulating GSK-3beta.[Pubmed:28320093]
Biomed Pharmacother. 2017 May;89:1262-1268.
Endoplasmic reticulum (ER) stress-induced chondrocyte apoptosis plays a critical role in osteoarthritis cartilage degeneration. Previous studies showed that 5,7,3',4'-tetramethoxyflavone (TMF) exhibited chondroprotective activity through inhibiting PGE2-induced ER stress and down regulating the expression of GSK-3beta. To further investigate the role of GSK-3beta in ER stress-induced chondrocytes apoptosis and the protective role of TMF, GSK-3beta siRNA and pcDNA3.1-myc-GSK-3beta were employed to knock down and overexpress GSK-3beta, respectively, in chondrocytes. Results showed that TM-induced ER stress significantly promoted chondrocytes apoptosis. These could be effectively reversed by GSK-3beta deficiency, while GSK-3beta overexpression significantly up regulated ER stress and increased chondrocytes apoptosis. In addition, TMF down regulated the expression of GSK-3beta and inhibited ER stress-induced chondrocytes apoptosis. Collectively, TMF is a potential natural compound with chondroprotective property through inhibition of ER stress-induced apoptosis with down regulation of GSK-3beta.
Demethylation of Polymethoxyflavones by Human Gut Bacterium, Blautia sp. MRG-PMF1.[Pubmed:28211698]
J Agric Food Chem. 2017 Mar 1;65(8):1620-1629.
Polymethoxyflavones (PMFs) were biotransformed to various demethylated metabolites in the human intestine by the PMF-metabolizing bacterium, Blautia sp. MRG-PMF1. Because the newly formed metabolites can have different biological activities, the pathways and regioselectivity of PMF bioconversion were investigated. Using an anaerobic in vitro study, 12 PMFs, 5,7-dimethoxyflavone (5,7-DMF), 5-hydroxy-7-methoxyflavone (5-OH-7-MF), 3,5,7-trimethoxyflavone (3,5,7-TMF), 5-hydroxy-3,7-dimethoxyflavone (5-OH-3,7-DMF), 5,7,4'-trimethoxyflavone (5,7,4'-TMF), 5-hydroxy-7,4'-dimethoxyflavone (5-OH-7,4'-DMF), 3,5,7,4'-tetramethoxyflavone (3,5,7,4'-TMF), 5-hydroxy-3,7,4'-trimethoxyflavone (5-OH-3,7,4'-TMF), 5,7,3',4'-tetramethoxyflavone (5,7,3',4'-TMF), 3,5,7,3',4'-pentamethoxyflavone (3,5,7,3',4'-PMF), 5-hydroxy-3,7,3',4'-tetramethoxyflavone (5-OH-3,7,3',4'-TMF), and 5,3'-dihydroxy-3,7,4'-trimethoxyflavone (5,3'-diOH-3,7,4'-TMF), were converted to chrysin, apigenin, galangin, kaempferol, luteolin, and quercetin after complete demethylation. The time-course monitoring of PMF biotransformations elucidated bioconversion pathways, including the identification of metabolic intermediates. As a robust flavonoid demethylase, regioselectivity of PMF demethylation generally followed the order C-7 > C-4' approximately C-3' > C-5 > C-3. PMF demethylase in the MRG-PMF1 strain was suggested as a Co-corrinoid methyltransferase system, and this was supported by the experiments utilizing other methyl aryl ether substrates and inhibitors.


