Rhaponticin 2''-O-gallateCAS# 94356-24-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 94356-24-8 | SDF | Download SDF |
| PubChem ID | 10325743 | Appearance | Powder |
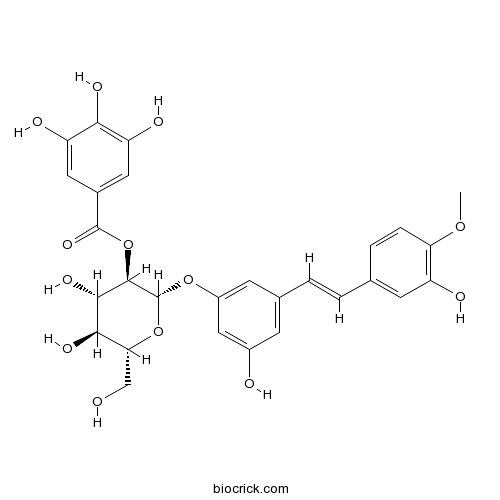
| Formula | C28H28O13 | M.Wt | 572.51 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | [(2S,3R,4S,5S,6R)-4,5-dihydroxy-2-[3-hydroxy-5-[(E)-2-(3-hydroxy-4-methoxyphenyl)ethenyl]phenoxy]-6-(hydroxymethyl)oxan-3-yl] 3,4,5-trihydroxybenzoate | ||
| SMILES | COC1=C(C=C(C=C1)C=CC2=CC(=CC(=C2)OC3C(C(C(C(O3)CO)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O)O)O | ||
| Standard InChIKey | DBHYLGJAFYCFGS-XJVIDBJFSA-N | ||
| Standard InChI | InChI=1S/C28H28O13/c1-38-21-5-4-13(8-18(21)31)2-3-14-6-16(30)11-17(7-14)39-28-26(25(36)24(35)22(12-29)40-28)41-27(37)15-9-19(32)23(34)20(33)10-15/h2-11,22,24-26,28-36H,12H2,1H3/b3-2+/t22-,24-,25+,26-,28-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Rhaponticin 2''-O-gallate showed inhibitory activity of NO production in lipopolysaccharide-activated macrophages, (IC50 = 11-69 microM). |
| Targets | NO |
| In vitro | Effects of stilbene constituents from rhubarb on nitric oxide production in lipopolysaccharide-activated macrophages.[Pubmed: 10714491 ]Bioorg Med Chem Lett. 2000 Feb 21;10(4):323-7.
|

Rhaponticin 2''-O-gallate Dilution Calculator

Rhaponticin 2''-O-gallate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7467 mL | 8.7335 mL | 17.4669 mL | 34.9339 mL | 43.6674 mL |
| 5 mM | 0.3493 mL | 1.7467 mL | 3.4934 mL | 6.9868 mL | 8.7335 mL |
| 10 mM | 0.1747 mL | 0.8733 mL | 1.7467 mL | 3.4934 mL | 4.3667 mL |
| 50 mM | 0.0349 mL | 0.1747 mL | 0.3493 mL | 0.6987 mL | 0.8733 mL |
| 100 mM | 0.0175 mL | 0.0873 mL | 0.1747 mL | 0.3493 mL | 0.4367 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-Hydroxy-2-(2-phenylethyl)chromone
Catalog No.:BCN8812
CAS No.:84294-90-6
- 6,7-Dimethoxy-2-phenethylchromone
Catalog No.:BCN8811
CAS No.:84294-87-1
- 6-Hydroxyluteolin 7-glucoside
Catalog No.:BCN8810
CAS No.:54300-65-1
- Volvalerenic acid A
Catalog No.:BCN8809
CAS No.:1247014-34-1
- Heishuixiecaoline A
Catalog No.:BCN8808
CAS No.:1469493-85-3
- Kalopanaxsaponin G
Catalog No.:BCN8806
CAS No.:171370-50-6
- Sterebin A
Catalog No.:BCN8802
CAS No.:107647-14-3
- Isoagarotetrol
Catalog No.:BCN8801
CAS No.:104060-61-9
- Norwogonin 5,7,8-trimethyl ether
Catalog No.:BCN8800
CAS No.:23050-38-6
- 24-Hydroxymomordicine III
Catalog No.:BCN8798
CAS No.:
- Pterodonoic acid
Catalog No.:BCN8797
CAS No.:62458-42-8
- Polygalin J
Catalog No.:BCN8796
CAS No.:
- 2,4,7-Trihydroxy-9,10-dihydrophenanthrene
Catalog No.:BCN8814
CAS No.:70205-52-6
- Chrysoeriol 7-apiosylglucoside
Catalog No.:BCN8815
CAS No.:33579-63-4
- 7,22,25-Stigmastatrienol
Catalog No.:BCN8816
CAS No.:14485-48-4
- 2-Hydroxy-1-Methoxyaporphine
Catalog No.:BCN8817
CAS No.:33770-27-3
- Aloe-emodin-3-(hydroxymethyl)-O-beta-D-glucopyranoside
Catalog No.:BCN8818
CAS No.:50488-89-6
- 5,7,3',4'-Tetramethoxyflavone
Catalog No.:BCN8821
CAS No.:855-97-0
- Combretol
Catalog No.:BCN8822
CAS No.:5084-19-5
- Ardisiacrispin B
Catalog No.:BCN8823
CAS No.:112766-96-8
- Ardisicrenoside A
Catalog No.:BCN8824
CAS No.:160824-52-2
- Chrysophanol 1-O-beta-tetraglucoside
Catalog No.:BCN8825
CAS No.:120181-08-0
- 11-Oxomogroside IV
Catalog No.:BCN8826
CAS No.:2096516-32-2
- 5'''-O-Feruloyl complanatoside B
Catalog No.:BCN8827
CAS No.:142473-98-1
Effects of stilbene constituents from rhubarb on nitric oxide production in lipopolysaccharide-activated macrophages.[Pubmed:10714491]
Bioorg Med Chem Lett. 2000 Feb 21;10(4):323-7.
Two new anthraquinone glucosides [chrysophanol 8-O-beta-D-(6'-galloyl)-glucopyranoside, aloe-emodin 1-O-beta-D-glucopyranoside] together with various known stilbenes and their glucosides, anthraquinone glucosides, and a naphthalene glucoside were isolated from the rhizome of Rheum undulatum L. Three stilbenes (rhapontigenin, piceatannol, resveratrol), a naphthalene glucoside (torachrysone 8-O-beta-D-glucopyranoside), and two stilbene glucoside gallates (rhaponticin 2''-O-gallate, rhaponticin 6''-O-gallate) showed inhibitory activity of NO production in lipopolysaccharide-activated macrophages, (IC50 = 11-69 microM). The oxygen functions (-OH,-OCH3) at the benzene ring were found to be essential to show the activity. Whereas, the glucoside moiety reduced the activity, while the alpha,beta-double bond did not affect the activity. Furthermore, the active stilbenes (rhapontigenin, piceatannol, resveratrol) inhibited iNOS induction.


