6-Hydroxyluteolin 7-glucosideCAS# 54300-65-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 54300-65-1 | SDF | Download SDF |
| PubChem ID | 185766 | Appearance | Powder |
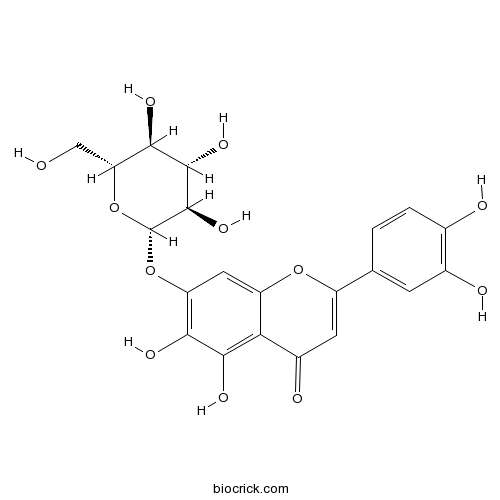
| Formula | C21H20O12 | M.Wt | 464.38 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 2-(3,4-dihydroxyphenyl)-5,6-dihydroxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxychromen-4-one | ||
| SMILES | C1=CC(=C(C=C1C2=CC(=O)C3=C(C(=C(C=C3O2)OC4C(C(C(C(O4)CO)O)O)O)O)O)O)O | ||
| Standard InChIKey | HYPKUHLLPBGDLF-IAAKTDFRSA-N | ||
| Standard InChI | InChI=1S/C21H20O12/c22-6-14-17(27)19(29)20(30)21(33-14)32-13-5-12-15(18(28)16(13)26)10(25)4-11(31-12)7-1-2-8(23)9(24)3-7/h1-5,14,17,19-24,26-30H,6H2/t14-,17-,19+,20-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| In vitro | Anti-Inflammatory and Antioxidant Activities from the Basolateral Fraction of Caco-2 Cells Exposed to a Rosmarinic Acid Enriched Extract.[Pubmed: 29345918 ]J Agric Food Chem. 2018 Feb 7;66(5):1167-1174.The potential use of Origanum majorana L. as a source of bioavailable phenolic compounds, specifically rosmarinic acid (RA), has been evaluated.
|
| Structure Identification | Phytochemistry, 1980, 19(8):1761-1766.New 6-hydroxyflavonoids and their methyl ethers and glycosides from Neurolaena oaxacana.[Reference: WebLink]Six new and nine known flavonoids were obtained from Neurolaena oaxacana.
|

6-Hydroxyluteolin 7-glucoside Dilution Calculator

6-Hydroxyluteolin 7-glucoside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1534 mL | 10.767 mL | 21.5341 mL | 43.0682 mL | 53.8352 mL |
| 5 mM | 0.4307 mL | 2.1534 mL | 4.3068 mL | 8.6136 mL | 10.767 mL |
| 10 mM | 0.2153 mL | 1.0767 mL | 2.1534 mL | 4.3068 mL | 5.3835 mL |
| 50 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8614 mL | 1.0767 mL |
| 100 mM | 0.0215 mL | 0.1077 mL | 0.2153 mL | 0.4307 mL | 0.5384 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Volvalerenic acid A
Catalog No.:BCN8809
CAS No.:1247014-34-1
- Heishuixiecaoline A
Catalog No.:BCN8808
CAS No.:1469493-85-3
- Kalopanaxsaponin G
Catalog No.:BCN8806
CAS No.:171370-50-6
- Sterebin A
Catalog No.:BCN8802
CAS No.:107647-14-3
- Isoagarotetrol
Catalog No.:BCN8801
CAS No.:104060-61-9
- Norwogonin 5,7,8-trimethyl ether
Catalog No.:BCN8800
CAS No.:23050-38-6
- 24-Hydroxymomordicine III
Catalog No.:BCN8798
CAS No.:
- Pterodonoic acid
Catalog No.:BCN8797
CAS No.:62458-42-8
- Polygalin J
Catalog No.:BCN8796
CAS No.:
- New biochemical 2
Catalog No.:BCN8792
CAS No.:
- Complanatoside C
Catalog No.:BCN8783
CAS No.:
- New biochemical 1
Catalog No.:BCN8781
CAS No.:
- 6,7-Dimethoxy-2-phenethylchromone
Catalog No.:BCN8811
CAS No.:84294-87-1
- 6-Hydroxy-2-(2-phenylethyl)chromone
Catalog No.:BCN8812
CAS No.:84294-90-6
- Rhaponticin 2''-O-gallate
Catalog No.:BCN8813
CAS No.:94356-24-8
- 2,4,7-Trihydroxy-9,10-dihydrophenanthrene
Catalog No.:BCN8814
CAS No.:70205-52-6
- Chrysoeriol 7-apiosylglucoside
Catalog No.:BCN8815
CAS No.:33579-63-4
- 7,22,25-Stigmastatrienol
Catalog No.:BCN8816
CAS No.:14485-48-4
- 2-Hydroxy-1-Methoxyaporphine
Catalog No.:BCN8817
CAS No.:33770-27-3
- Aloe-emodin-3-(hydroxymethyl)-O-beta-D-glucopyranoside
Catalog No.:BCN8818
CAS No.:50488-89-6
- 5,7,3',4'-Tetramethoxyflavone
Catalog No.:BCN8821
CAS No.:855-97-0
- Combretol
Catalog No.:BCN8822
CAS No.:5084-19-5
- Ardisiacrispin B
Catalog No.:BCN8823
CAS No.:112766-96-8
- Ardisicrenoside A
Catalog No.:BCN8824
CAS No.:160824-52-2
The flavonoids of Tanacetum parthenium and T. vulgare and their anti-inflammatory properties.[Pubmed:10382317]
Phytochemistry. 1999 Jun;51(3):417-23.
The lipophilic flavonoids in leaf and flower of Tanacetum parthenium and T. vulgaris have been compared. While those of T. parthenium are methyl ethers of the flavonols 6-hydroxykaempferol and quercetagetin, the surface flavonoids of T. vulgare are methyl ethers of the flavones scutellarein and 6-hydroxyluteolin. Apigenin and two flavone glucuronides are surprisingly present in glandular trichomes on the lower epidermis of the ray florets of T. parthenium. The opportunity has been taken to revise the structures of the four 6-hydroxyflavonol methyl ethers of T. parthenium based on NMR measurements. These are now shown to be uniformly 6- rather than 7-O-methylated. Tanetin, previously thought to be a new structure, is now formulated as the known 6-hydroxykaempferol 3,6,4'-trimethyl ether. The vacuolar flavonoids of both plants are dominated by the presence of apigenin and luteolin 7-glucuronides; nine other glycosides were present, including the uncommon 6-Hydroxyluteolin 7-glucoside in T. vulgare. When the major flavonol and flavone methyl ethers of the two plants were tested pharmacologically, they variously inhibited the major pathways of arachidonate metabolism in leukocytes. There were significant differences in potency, with the tansy 6-hydroxyflavones less active than the feverfew 6-hydroxyflavonols as inhibitors of cyclo-oxygenase and 5-lipoxygenase.


