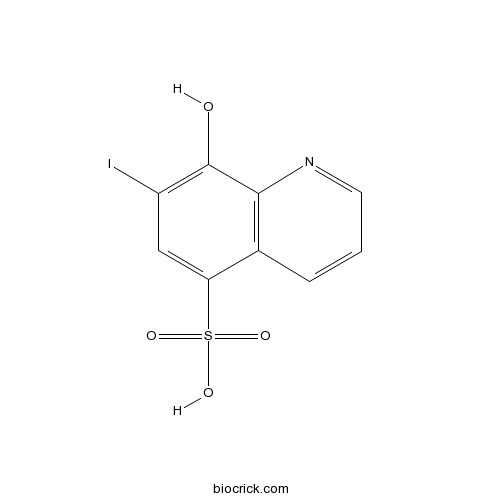
8-Hydroxy-7-iodo-5-quinolinesulfonic acidCAS# 547-91-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 547-91-1 | SDF | Download SDF |
| PubChem ID | 11043 | Appearance | Powder |
| Formula | C9H6INO4S | M.Wt | 351 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 8-hydroxy-7-iodoquinoline-5-sulfonic acid | ||
| SMILES | C1=CC2=C(C(=C(C=C2S(=O)(=O)O)I)O)N=C1 | ||
| Standard InChIKey | ZBJWWKFMHOAPNS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C9H6INO4S/c10-6-4-7(16(13,14)15)5-2-1-3-11-8(5)9(6)12/h1-4,12H,(H,13,14,15) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

8-Hydroxy-7-iodo-5-quinolinesulfonic acid Dilution Calculator

8-Hydroxy-7-iodo-5-quinolinesulfonic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.849 mL | 14.245 mL | 28.49 mL | 56.9801 mL | 71.2251 mL |
| 5 mM | 0.5698 mL | 2.849 mL | 5.698 mL | 11.396 mL | 14.245 mL |
| 10 mM | 0.2849 mL | 1.4245 mL | 2.849 mL | 5.698 mL | 7.1225 mL |
| 50 mM | 0.057 mL | 0.2849 mL | 0.5698 mL | 1.1396 mL | 1.4245 mL |
| 100 mM | 0.0285 mL | 0.1425 mL | 0.2849 mL | 0.5698 mL | 0.7123 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4'-Benzyloxyacetophenone
Catalog No.:BCC8698
CAS No.:54696-05-8
- 2-(Acetylamino)-3-phenyl-2-propenoic acid
Catalog No.:BCN1420
CAS No.:5469-45-4
- 1-O-Methyljatamanin D
Catalog No.:BCN6671
CAS No.:54656-47-2
- ML 204
Catalog No.:BCC6272
CAS No.:5465-86-1
- 2-Amino-4-methoxybenzothiazole
Catalog No.:BCC8532
CAS No.:5464-79-9
- Boc-Hyp(Bzl)-OH.DCHA
Catalog No.:BCC3253
CAS No.:54631-81-1
- Diethyl 2-acetamido-2-phenethylmalonate
Catalog No.:BCC8940
CAS No.:5463-92-3
- URB597
Catalog No.:BCC2324
CAS No.:546141-08-6
- Boc-Lys(2-Cl-Z)-OH
Catalog No.:BCC3416
CAS No.:54613-99-9
- Columbin
Catalog No.:BCN2622
CAS No.:546-97-4
- α-Thujone
Catalog No.:BCC8271
CAS No.:546-80-5
- Alantolactone
Catalog No.:BCN1033
CAS No.:546-43-0
- Dodecanoic acid ingenol ester
Catalog No.:BCN8291
CAS No.:54706-70-6
- 20-Deoxyingenol
Catalog No.:BCN3770
CAS No.:54706-99-9
- 4-(4-Hydroxyphenyl)-2-butanone
Catalog No.:BCN6797
CAS No.:5471-51-2
- Fluvoxamine
Catalog No.:BCC4214
CAS No.:54739-18-3
- JTE 013
Catalog No.:BCC7348
CAS No.:547756-93-4
- BMS-345541
Catalog No.:BCC1423
CAS No.:547757-23-3
- Hypericin
Catalog No.:BCN5977
CAS No.:548-04-9
- Roemerine
Catalog No.:BCN8236
CAS No.:548-08-3
- Isoginkgetin
Catalog No.:BCN2320
CAS No.:548-19-6
- Isolariciresinol
Catalog No.:BCN5727
CAS No.:548-29-8
- Oxysanguinarine
Catalog No.:BCN8100
CAS No.:548-30-1
- Cornin
Catalog No.:BCN5008
CAS No.:548-37-8
New insights into the mechanism of antifungal action of 8-hydroxyquinolines.[Pubmed:30662305]
Saudi Pharm J. 2019 Jan;27(1):41-48.
The 8-hydroxyquinoline core is a privileged scaffold for drug design explored to afford novel derivatives endowed with biological activity. Our research aimed at clarifying the antifungal mechanism of action of clioquinol, 8-hydroxy-5-quinolinesulfonic acid, and 8-Hydroxy-7-iodo-5-quinolinesulfonic acid (three 8-hydroxyquinoline derivatives). The antifungal mode of action of these derivatives on Candida spp. and dermatophytes was investigated using sorbitol protection assay, cellular leakage effect, ergosterol binding assay, and scanning electron microscopy. Clioquinol damaged the cell wall and inhibited the formation of pseudohyphae by C. albicans. The 8-hydroxy-5-quinolinesulfonic acid derivatives compromised the functional integrity of cytoplasmic membranes. To date no similar report was found about the antifungal mechanism of 8-hydroxyquinolines. These results, combined with the broad antifungal spectrum already demonstrated previously, reinforce the potential of 8-hydroxyquinolines for the development of new drugs.
Evaluation of 8-Hydroxyquinoline Derivatives as Hits for Antifungal Drug Design.[Pubmed:28159993]
Med Mycol. 2017 Oct 1;55(7):763-773.
Clioquinol is an 8-hydroxyquinoline derivative that was widely used from the 1950s to 1970s as an oral antiparasitic agent. In 1970, the oral forms were withdrawn from the market due to reports of toxicity, but topical formulations for antifungal treatment remained available. Thus, the purpose of this study was to evaluate the toxicity, anti-Candida and antidermatophyte activity and to determine pharmacodynamic characteristics of clioquinol and other 8-hydroxyquinoline derivatives (8-hydroxy-5-quinolinesulfonic acid and 8-Hydroxy-7-iodo-5-quinolinesulfonic acid). Antifungal activity was tested by broth microdilution and the fungicidal or fungistatic effect was checked by a time-kill assay. Permeation and histopathological evaluation were performed in Franz diffusion cells with ear skin of pigs and examined under light microscopy. An HET-CAM test was used to determine the potential irritancy. The three compounds were active against all isolates showing anti-Candida and antidermatophyte activity, with MIC ranges of 0.031-2 mug/ml, 1-512 mug/ml, and 2-1024 mug/ml for clioquinol, 8-hydroxy-5-quinolinesulfonic acid, and 8-Hydroxy-7-iodo-5-quinolinesulfonic acid, respectively. All compounds showed fungistatic effect for Candida, 8-hydroxy-5-quinolinesulfonic acid, and 8-Hydroxy-7-iodo-5-quinolinesulfonic acid showed a fungicidal effect for M. canis and T. mentagrophytes, and clioquinol showed a fungicidal effect only for T. mentagrophytes. Furthermore, they presented a fungicidal effect depending on the time and concentration. The absence of lesions was observed in histopathological evaluation and no compound was irritating. Moreover, clioquinol and 8-hydroxy-5-quinolinesulfonic acid accumulated in the epithelial tissue, and 8-Hydroxy-7-iodo-5-quinolinesulfonic acid had a high degree of permeation. In conclusion, 8-hydroxyquinoline derivatives showed antifungal activity and 8-hydroxy-5-quinolinesulfonic acid demonstrated the potential for antifungal drug design.
Aluminum polymers formed following alum treatment of lake water.[Pubmed:20825969]
Chemosphere. 2010 Nov;81(7):832-6.
Alum (aluminum sulfate) is increasingly being used in lake management to control internal recycling of phosphorus from bottom sediments. Alum added to water undergoes rapid hydrolysis reactions, forming an amorphous Al(OH)3 floc with a high capacity for sorption of phosphorus. While it is known that the Al(OH)3 floc transforms over time to more ordered microcrystalline and crystalline gibbsite phases, there remains an incomplete understanding of the forms of Al present immediately following alum addition to lake water. A laboratory study was thus undertaken to evaluate the forms of Al present following alum addition using ferron (8-Hydroxy-7-iodo-5-quinolinesulfonic acid) timed-colorimetric and 27Al-NMR measurements. A polymeric Al species with moderate reactivity with ferron (Alb2) was initially formed, although it rapidly transformed to a less ferron-reactive colloidal form (Alc) and also decomposed at low alum doses to monomeric Al (Ala) in response to pH increases associated with outgassing of CO2. The Ala fraction in these solutions could be adequately estimated based upon measured pH assuming Al solubility was controlled by an amorphous Al(OH)3 phase. Al13 was inferred from ferron measurements to be present, but only at quite low concentrations in the alum-treated waters.
Six spectroscopic methods for detection of oxidants in urine: implication in differentiation of normal and adulterated urine.[Pubmed:15516320]
J Anal Toxicol. 2004 Oct;28(7):599-608.
Six separate methods to detect oxidants in urine were developed. The presence of the oxidants was established by initial oxidation of ferrous to ferric ion and detecting the ferric by chromogenic oxidation or complex formation. The reagents for chromogenic oxidation were N,N-dimethylamoino-1,4-phenylenediamine (DMPDA), 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS), and 2-amino-p-cresol (APC), and the reagents for the chromogenic complex were xylenol orange (XO), 8-Hydroxy-7-iodo-5-quinolinesulfonic acid (HIQSA), and 4,5-dihydroxy-1,3-benzene-di-sulfonic acid (HBSA). All methods showed comparable results when tested for ferric, chromate, permanganate, oxychloride, hydrogen peroxide, oxone, tert-butylhydroperoxide, and cumenehydroperoxide at a concentration of 1.0 mmol/L in water (CV < 7%). The nitrite results are comparable only with DMPDA and APC. Periodate responded to the highest oxidation number (ON = 8) by chromogenic oxidation but lowest (ON = 2) by the chromogenic complex. The iodate responded only to the chromogenic oxidation with ON = 6. The linearity of the procedures was established by chromate in water. The linear concentrations were 0.09-12.00 mE/L for DMPDA, ABTS, APC, and HBSA and 0.09-6.00 mE/L for XO and HIQSA. In all methods, the correlation coefficients were > or = 0.9991 and precisions were within +/- 5.6%. The methods were used to test oxidants in 238 urine specimens. The chromate at 3.0 mE/L in water was used as standard. The correlation coefficients of 0.9600-0.9853 and the ANOVA test (F = 0.90, F(critical) = 2.22 at P(0.48)) indicated that the methods correlated well. The median concentration of oxidants in the specimens was 0.21 mE/L with an average and standard deviation of 0.62 +/- 1.19 (range 0.04-8.83 mE/L). When Grubbs' statistical test was applied to the specimen results, no specimen was found to be outlier or could be considered as adulterated. The Grubbs' test also revealed that the threshold concentration to identify urine adulteration was 29 mE/L at confidence level of 99%.
Assessing the Rhizotoxicity of the Aluminate Ion, Al(OH)(4).[Pubmed:16667665]
Plant Physiol. 1990 Aug;93(4):1620-5.
Dissolved aluminum (III) in acidic soils or culture media is often rhizotoxic (inhibitory to root elongation). Alkaline solutions of Al are also sometimes rhizotoxic, and for that reason toxicity has been attributed to the aluminate ion, Al(OH)(4) (-). In the present study, seedlings of wheat (Triticum aestivum L. cv Tyler) and red clover (Trifolium pratense L. cv Kenland) were cultured in aerated aluminate solutions at pH 8.0 to 8.9. The bulk phases of these solutions were free of reactive polynuclear hydroxy-Al (including the extremely toxic species AlO(4)Al(12)[OH](24)[H(2)O](7+) (12) [Al(13)]) according to the ferron (8-Hydroxy-7-iodo-5-quinolinesulfonic acid) assay. At an aluminate concentration of 25 micromolar (23 micromolar activity) and a pH of 8, root elongation was less than 40% of Al-free controls, but at pH 8.9 elongation was 100% of controls. The hypothesis is offered that aluminate is nontoxic and that the inhibition at lower pH values is attributable to Al(13) postulated to have formed in the acidic free space of the roots where the ratio /{Al(3+)/}//{H(+)/}(3) may rise above 10(10). At this value hydroxy-Al in over-saturated, alkaline solutions begins to undergo rapid conversion to polynuclear species.


