A-803467NaV1.8 channel blocker,potent and selective CAS# 944261-79-4 |
- KX2-391 dihydrochloride
Catalog No.:BCC1686
CAS No.:1038395-65-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Bosutinib (SKI-606)
Catalog No.:BCC1167
CAS No.:380843-75-4
- Dasatinib hydrochloride
Catalog No.:BCC1517
CAS No.:854001-07-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 944261-79-4 | SDF | Download SDF |
| PubChem ID | 16038374 | Appearance | Powder |
| Formula | C19H16ClNO4 | M.Wt | 357.79 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (139.75 mM; Need ultrasonic) | ||
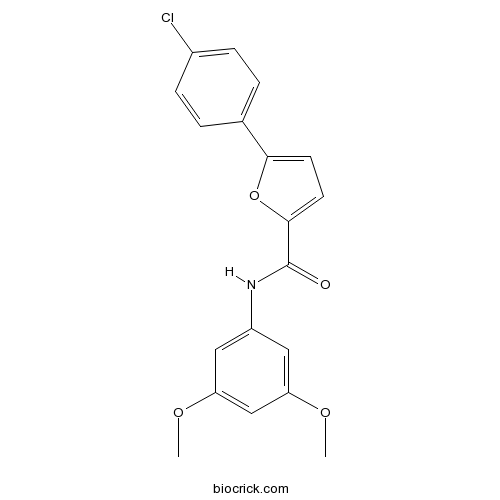
| Chemical Name | 5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-carboxamide | ||
| SMILES | COC1=CC(=CC(=C1)NC(=O)C2=CC=C(O2)C3=CC=C(C=C3)Cl)OC | ||
| Standard InChIKey | VHKBTPQDHDSBSP-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H16ClNO4/c1-23-15-9-14(10-16(11-15)24-2)21-19(22)18-8-7-17(25-18)12-3-5-13(20)6-4-12/h3-11H,1-2H3,(H,21,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective blocker of NaV1.8 channels (IC50 values are 8, 2450, 6740, 7340 and 7380 nM for hNaV1.8, hNaV1.3, hNaV1.7, hNaV1.5 and hNaV1.2 channels respectively). Shows no significant activity against TRPV1, P2X2/3, CaV2.2 and KCNQ2/3 channels. Antinociceptive; potently attenuates mechanical allodynia in two models of neuropathic pain following i.p. administration. |

A-803467 Dilution Calculator

A-803467 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7949 mL | 13.9747 mL | 27.9494 mL | 55.8987 mL | 69.8734 mL |
| 5 mM | 0.559 mL | 2.7949 mL | 5.5899 mL | 11.1797 mL | 13.9747 mL |
| 10 mM | 0.2795 mL | 1.3975 mL | 2.7949 mL | 5.5899 mL | 6.9873 mL |
| 50 mM | 0.0559 mL | 0.2795 mL | 0.559 mL | 1.118 mL | 1.3975 mL |
| 100 mM | 0.0279 mL | 0.1397 mL | 0.2795 mL | 0.559 mL | 0.6987 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A-803467 is a potent and selective blocker of Nav1.8 sodium channel with IC50 value of 8nM [1].
A-803467 is a sodium channel blocker with high affinity and selectivity. It blocks the human Nav1.8 channels with IC50 value of 79nM. It also blocks the recombinant rat Nav1.8 channels with IC50 value of 45nM when the holding potential is -40mV. A-803467 is highly selective against Nav1.8 since the inhibition of Nav1.8 is 300- to 1000-fold more potent than of NaV1.2, NaV1.3, NaV1.5, and NaV1.7. It is found that A-803467 effectively suppresses the evoked and spontaneous action potential firing in DRG neurons in which TTX-R currents play a remarkable role. Furthermore, the selective blockade of Nav1.8 channels results in a significant reduction in nociceptive sensitivity. A-803467 potently attenuates mechanical allodynia in models of neuropathic pain. It also potently reduces thermal hyperalgesia in the CFA model of inflammatory pain [1].
References:
[1] Jarvis M F, Honore P, Shieh C C, et al. A-803467, a potent and selective Nav1. 8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proceedings of the National Academy of Sciences, 2007, 104(20): 8520-8525.
- MJ 15
Catalog No.:BCC7852
CAS No.:944154-76-1
- Peficitinb (ASP015K, JNJ-54781532)
Catalog No.:BCC6503
CAS No.:944118-01-8
- PG 106
Catalog No.:BCC6330
CAS No.:944111-22-2
- Isomartynoside
Catalog No.:BCN4497
CAS No.:94410-22-7
- Tetrachlorohydroquinone dimethyl ether
Catalog No.:BCN1301
CAS No.:944-78-5
- Dimethylmatairesinol
Catalog No.:BCN4496
CAS No.:943989-68-2
- BMS-303141
Catalog No.:BCC4097
CAS No.:943962-47-8
- Ophiopogoside A
Catalog No.:BCC8346
CAS No.:943914-99-6
- Sebiferenic acid
Catalog No.:BCN4495
CAS No.:94390-09-7
- Isomucronulatol 7-O-glucoside
Catalog No.:BCN3845
CAS No.:94367-43-8
- Methylnissolin-3-O-glucoside
Catalog No.:BCN3829
CAS No.:94367-42-7
- Piceatannol 3'-O-glucoside
Catalog No.:BCN2874
CAS No.:94356-26-0
- QS 11
Catalog No.:BCC7648
CAS No.:944328-88-5
- JNJ 28871063 hydrochloride
Catalog No.:BCC7662
CAS No.:944342-90-9
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
- Bacopaside X
Catalog No.:BCC8126
CAS No.:94443-88-6
- Syzalterin
Catalog No.:BCN3969
CAS No.:94451-48-6
- Cromakalim
Catalog No.:BCC7038
CAS No.:94470-67-4
- Lck inhibitor 2
Catalog No.:BCC1690
CAS No.:944795-06-6
- Rhuscholide A
Catalog No.:BCN4498
CAS No.:944804-58-4
- Isodaphnoretin B
Catalog No.:BCN6913
CAS No.:944824-29-7
- Decernotinib(VX-509)
Catalog No.:BCC6456
CAS No.:944842-54-0
- Angelic anhydride
Catalog No.:BCN3411
CAS No.:94487-74-8
- 2'-Acetylacteoside
Catalog No.:BCN3409
CAS No.:94492-24-7
The effects of A-803467 on cardiac Nav1.5 channels.[Pubmed:25701724]
Eur J Pharmacol. 2015 May 5;754:52-60.
A-803467 is a selective Nav1.8 blocker, but its mechanism of action at cardiac sodium channels is uncertain. Thus, we investigated the mechanistic effects of A-803467 on cardiac sodium channels in isolated mouse ventricular myocytes and in human embryonic kidney 293 (HEK293) cell lines that transiently expressed Nav1.5/SCN5A, the predominant cardiac sodium channel. At 0.3muM and greater, A-803467 blocked cardiac sodium currents in a dose-dependent manner in both ventricular myocytes and in SCN5A-expressing HEK293 cell lines. In both models, the drug caused significant depolarizing shifts at the conductance voltage relationship midpoint, hyperpolarizing shifts in voltage-dependent channel inactivation, and slower recovery from inactivation. Also, the drug reduced sodium current amplitude in a frequency-dependent manner, and blocked late sodium currents, accelerated inactivation, and enhanced the intermediate inactivation state. Our results provide strong evidence that A-803467 affects multiple biophysical characteristics of the canonical cardiac Nav1.5 channel and our data can be used to study potential applications of A-803467 as an antiarrhythmic drug.
A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo.[Pubmed:26515463]
Oncotarget. 2015 Nov 17;6(36):39276-91.
ATP-binding cassette subfamily G member 2 (ABCG2) is a member of the ABC transporter superfamily proteins, which has been implicated in the development of multidrug resistance (MDR) in cancer, apart from its physiological role to remove toxic substances out of the cells. The diverse range of substrates of ABCG2 includes many antineoplastic agents such as topotecan, doxorubicin and mitoxantrone. ABCG2 expression has been reported to be significantly increased in some solid tumors and hematologic malignancies, correlated to poor clinical outcomes. In addition, ABCG2 expression is a distinguishing feature of cancer stem cells, whereby this membrane transporter facilitates resistance to the chemotherapeutic drugs. To enhance the chemosensitivity of cancer cells, attention has been focused on MDR modulators. In this study, we investigated the effect of a tetrodotoxin-resistant sodium channel blocker, A-803467 on ABCG2-overexpressing drug selected and transfected cell lines. We found that at non-toxic concentrations, A-803467 could significantly increase the cellular sensitivity to ABCG2 substrates in drug-resistant cells overexpressing either wild-type or mutant ABCG2. Mechanistic studies demonstrated that A-803467 (7.5 muM) significantly increased the intracellular accumulation of [(3)H]-mitoxantrone by inhibiting the transport activity of ABCG2, without altering its expression levels. In addition, A-803467 stimulated the ATPase activity in membranes overexpressed with ABCG2. In a murine model system, combination treatment of A-803467 (35 mg/kg) and topotecan (3 mg/kg) significantly inhibited the tumor growth in mice xenografted with ABCG2-overexpressing cancer cells. Our findings indicate that a combination of A-803467 and ABCG2 substrates may potentially be a novel therapeutic treatment in ABCG2-positive drug resistant cancers.
Additive antinociceptive effects of the selective Nav1.8 blocker A-803467 and selective TRPV1 antagonists in rat inflammatory and neuropathic pain models.[Pubmed:19070548]
J Pain. 2009 Mar;10(3):306-15.
UNLABELLED: Evidence implicating Nav1.8 and TRPV1 ion channels in various chronic pain states is extensive. In this study, we used isobolographic analysis to examine the in vivo effects of the combination of the Nav1.8 blocker A-803467 [5-(4-Chloro-phenyl)-furan-2-carboxylic acid (3,5-dimethoxy-phenyl)-amide] with 2 structurally distinct TRPV1 antagonists, A-840257 [1-(1H-Indazol-4-yl)-3-([R]-4-piperidin-1-yl-indan-1-yl)-urea] or A-425619 [1-Isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea]. The antinociceptive effects of the Nav1.8 blocker alone and in combination with each TRPV1 antagonist were examined in an inflammatory (complete Freund's adjuvant, CFA) and a neuropathic (spinal nerve ligation, SNL) pain model after systemic (intraperitoneal) administration. Alone, A-803467 was efficacious in both CFA and SNL models with ED(50) values of 70 (54.2 to 95.8) mg/kg and 70 (38.1 to 111.9) mg/kg, respectively. The ED(50) values of the TRPV1 antagonists A-840257 and A-425619 alone in the CFA model were 10 (3.6 to 14.9) mg/kg and 43 (24.1 to 62.2) mg/kg, respectively; both were without significant effect in the SNL model. A series of experiments incorporating 1:1, 3:1, or 0.3:1 ED(50) dose-ratio combinations of A-840257 and A-803467, or A-425619 and A-803467 were performed in both pain models, and the effective doses of mixtures that produced 50% antinociception (ED(50, mix)) were determined by isobolographic analysis. The ED(50, mix) in each case was not found to be statistically different than ED(50, add), the theoretical ED(50) calculated assuming additive effects. These data demonstrate that Nav1.8 blockers and TRPV1 antagonists administered in combination produce an additive effect in rat pain models. Using such a combination strategy to produce analgesia may potentially provide an improved therapeutic separation from unwanted in vivo side effects associated with blockade of either Nav1.8 or TRPV1 alone. PERSPECTIVE: In this report, effects of coadministration of TRPV1 antagonists and A-803467, a Nav1.8 blocker, were investigated in preclinical rodent models of neuropathic and inflammatory pain. The 2 classes of novel antinociceptive agents produced an additive interaction in attenuating CFA-induced thermal hyperalgesia, providing a rationale for their use as a combination strategy in the clinic for treating inflammatory pain.
A selective Nav1.8 sodium channel blocker, A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], attenuates spinal neuronal activity in neuropathic rats.[Pubmed:18089840]
J Pharmacol Exp Ther. 2008 Mar;324(3):1204-11.
We have recently reported that systemic delivery of A-803467 [5-(4-chlorophenyl-N-(3,5-dimethoxyphenyl)furan-2-carboxamide], a selective Na(v)1.8 sodium channel blocker, reduces behavioral measures of chronic pain. In the current study, the effects of A-803467 on evoked and spontaneous firing of wide dynamic range (WDR) neurons were measured in uninjured and rats with spinal nerve ligations (SNLs). Administration of A-803467 (10-30 mg/kg i.v.) reduced mechanically evoked (10-g von Frey hair) and spontaneous WDR neuronal activity in SNL rats. In uninjured rats, A-803467 (20 mg/kg i.v.) transiently reduced evoked but not spontaneous firing of WDR neurons. The systemic effects of A-803467 in SNL rats were not altered by spinal transection or by systemic pretreatment with the transient receptor potential vanilloid type 1 (TRPV1) receptor agonist, resiniferatoxin, at doses that impair the function of TRPV1-expressing fibers. To determine sites of action, A-803467 was administered into spinal tissue, into the uninjured L4 dorsal root ganglion (DRG), or into the neuronal receptive field. Injections of A-803467 into the L4 DRG (30-100 nmol/1 mul) or into the hindpaw receptive field (300 nmol/50 mul) reduced evoked but not spontaneous WDR firing. In contrast, intraspinal (50-150 nmol/0.5 mul) injection of A-803467 decreased both evoked and spontaneous discharges of WDR neurons. Thus, Na(v)1.8 sodium channels on the cell bodies/axons within the L4 DRG as well as on peripheral and central terminals of primary afferent neurons regulate the inflow of low-intensity mechanical signals to spinal WDR neurons. However, Na(v)1.8 sodium channels on central terminals seem to be key to the modulation of spontaneous firing in SNL rats.
Discovery and biological evaluation of 5-aryl-2-furfuramides, potent and selective blockers of the Nav1.8 sodium channel with efficacy in models of neuropathic and inflammatory pain.[Pubmed:18176998]
J Med Chem. 2008 Feb 14;51(3):407-16.
Nav1.8 (also known as PN3) is a tetrodotoxin-resistant (TTx-r) voltage-gated sodium channel (VGSC) that is highly expressed on small diameter sensory neurons and has been implicated in the pathophysiology of inflammatory and neuropathic pain. Recent studies using an Nav1.8 antisense oligonucleotide in an animal model of chronic pain indicated that selective blockade of Nav1.8 was analgesic and could provide effective analgesia with a reduction in the adverse events associated with nonselective VGSC blocking therapeutic agents. Herein, we describe the preparation and characterization of a series of 5-substituted 2-furfuramides, which are potent, voltage-dependent blockers (IC50 < 10 nM) of the human Nav1.8 channel. Selected derivatives, such as 7 and 27, also blocked TTx-r sodium currents in rat dorsal root ganglia (DRG) neurons with comparable potency and displayed >100-fold selectivity versus human sodium (Nav1.2, Nav1.5, Nav1.7) and human ether-a-go-go (hERG) channels. Following systemic administration, compounds 7 and 27 dose-dependently reduced neuropathic and inflammatory pain in experimental rodent models.
Painful research: identification of a small-molecule inhibitor that selectively targets Nav1.8 sodium channels.[Pubmed:17827438]
Mol Interv. 2007 Aug;7(4):192-5, 180.
Voltage-gated sodium channels in nociceptive neurons are attractive targets for novel pain therapeutics. Although drugs that target voltage-gated sodium channels have proven value as pain therapeutics, the drugs that are currently available are non-specific sodium channel inhibitors, which limit their usefulness. Recently, a selective small-molecule inhibitor of Na(v)1.8, a voltage-gated sodium channel isoform that participates in peripheral pain mechanisms, has been developed. This exciting new compound shows efficacy in several animal models of pain and is anticipated to be only the first of many new isoform-specific sodium channel blockers.


