Decernotinib(VX-509)Selective and orally active JAK3 inhibitor CAS# 944842-54-0 |
- Naphthoquine phosphate
Catalog No.:BCC1784
CAS No.:173531-58-3
- Mefloquine hydrochloride
Catalog No.:BCC1737
CAS No.:51773-92-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 944842-54-0 | SDF | Download SDF |
| PubChem ID | 59422203 | Appearance | Powder |
| Formula | C18H19F3N6O | M.Wt | 392.38 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | VX-509; VRT-831509 | ||
| Solubility | >16.95mg/mL in DMSO | ||
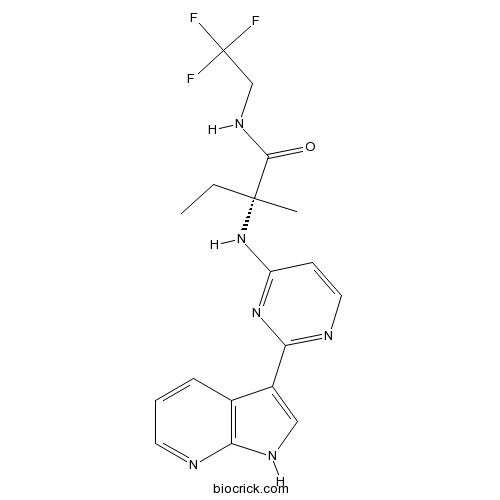
| Chemical Name | (2R)-2-methyl-2-[[2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl]amino]-N-(2,2,2-trifluoroethyl)butanamide | ||
| SMILES | CCC(C)(C(=O)NCC(F)(F)F)NC1=NC(=NC=C1)C2=CNC3=C2C=CC=N3 | ||
| Standard InChIKey | ASUGUQWIHMTFJL-QGZVFWFLSA-N | ||
| Standard InChI | InChI=1S/C18H19F3N6O/c1-3-17(2,16(28)25-10-18(19,20)21)27-13-6-8-23-15(26-13)12-9-24-14-11(12)5-4-7-22-14/h4-9H,3,10H2,1-2H3,(H,22,24)(H,25,28)(H,23,26,27)/t17-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Decernotinib is a potent, orally active JAK3 inhibitor, with Kis of 2.5, 11, 13 and 11 nM for JAK3, JAK1, JAK2, and TYK2, respectively.In Vitro:Decernotinib (VX-509) is a potent JAK3 inhibitor, with Kis of 2.5, 11, 13 and 11 nM for JAK3, JAK1, JAK2, and TYK2, respectively. Decernotinib potently blocks T-cell proliferation with a mean IC50 of 170 ± 101 nM, and inhibits IL-2-stimulated T-cell proliferation (IC50, 140 and 400 nM). VX-509 is also cytotoxic to B-cell in response to CD40L and IL-4 (IC50, 50 nM)[1].In Vivo:Decernotinib (VX-509, 10, 25, or 50 mg/kg, p.o.) significantly and dose-dependently inhibits the increases in ankle diameter and paw weight occuring in response to collagen injections in rats. Decernotinib potently alleviates cartilage damage and bone resorption in rats. Decernotinib (10, 25, or 50 mg/kg, p.o., b.i.d.) suppresses ear edema in a mouse model of delayed-type hypersensitivity[1]. References: | |||||

Decernotinib(VX-509) Dilution Calculator

Decernotinib(VX-509) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5485 mL | 12.7427 mL | 25.4855 mL | 50.971 mL | 63.7137 mL |
| 5 mM | 0.5097 mL | 2.5485 mL | 5.0971 mL | 10.1942 mL | 12.7427 mL |
| 10 mM | 0.2549 mL | 1.2743 mL | 2.5485 mL | 5.0971 mL | 6.3714 mL |
| 50 mM | 0.051 mL | 0.2549 mL | 0.5097 mL | 1.0194 mL | 1.2743 mL |
| 100 mM | 0.0255 mL | 0.1274 mL | 0.2549 mL | 0.5097 mL | 0.6371 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Decernotinib(VX-509)is a novel, potent and selective inhibitor of JAK3 with Ki value of 2 nM [1].
The Janus kinase family consists of four members: JAK1, JAK2, JAK3, and TYK2. Janus kinase 3 (JAK3) is mainly expressed in lymphocytes, which are cells important to the immune response associated with many diseases, including rheumatoid arthritis (RA) [1].
Decernotinib(VX-509)is a novel, potent, selective and orally available JAK3 inhibitor for the treatment of autoimmune diseases. VX-509 inhibited JAK1, JAK2, JAK3, and TYK2 with Ki values of 11, 13, 2 and 11 nM, respectively. In HT-2 or TF-1 cells, VX-509 inhibited JAK3/1- or JAK2-mediated phosphorylation of STAT5 following stimulation with IL-2 or GMCSF with IC50 values of 99 and 2600 nM, respectively [1].
In the rat host versus graft (HvG) model, VX-509 at 50 mg/kg bid or 100 mg/kg qd significantly and dose-dependently inhibited popletial lymph node (PLN) hyperplasia by 66% and 94%, respectively, relative to cyclosporin A (CsA). VX-509 also significantly reduced CD25 expression. These results suggested that VX-509 showed significant dose-dependent immunosuppressive activity and effectively inhibited T-cell activation [1]. In the rat collagen-induced arthritis model, VX-509 dose-dependently reduced ankle swelling and paw weight, and improved paw histopathology scores. In oxazolone-induced delayed-type hypersensitivity mouse model, VX-509 reduced the T cell-mediated inflammatory response in skin [2].
References:
[1]. Farmer LJ, Ledeboer MW, Hoock T, et al. Discovery of VX-509 (Decernotinib): A Potent and Selective Janus Kinase 3 Inhibitor for the Treatment of Autoimmune Diseases. J Med Chem, 2015, 58(18): 7195-7216.
[2]. Mahajan S, Hogan JK, Shlyakhter D, et al. VX-509 (decernotinib) is a potent and selective janus kinase 3 inhibitor that attenuates inflammation in animal models of autoimmune disease. J Pharmacol Exp Ther, 2015, 353(2): 405-414.
- Isodaphnoretin B
Catalog No.:BCN6913
CAS No.:944824-29-7
- Rhuscholide A
Catalog No.:BCN4498
CAS No.:944804-58-4
- Lck inhibitor 2
Catalog No.:BCC1690
CAS No.:944795-06-6
- Cromakalim
Catalog No.:BCC7038
CAS No.:94470-67-4
- Syzalterin
Catalog No.:BCN3969
CAS No.:94451-48-6
- Bacopaside X
Catalog No.:BCC8126
CAS No.:94443-88-6
- BKM120
Catalog No.:BCC1279
CAS No.:944396-07-0
- JNJ 28871063 hydrochloride
Catalog No.:BCC7662
CAS No.:944342-90-9
- QS 11
Catalog No.:BCC7648
CAS No.:944328-88-5
- A-803467
Catalog No.:BCC5075
CAS No.:944261-79-4
- MJ 15
Catalog No.:BCC7852
CAS No.:944154-76-1
- Peficitinb (ASP015K, JNJ-54781532)
Catalog No.:BCC6503
CAS No.:944118-01-8
- Angelic anhydride
Catalog No.:BCN3411
CAS No.:94487-74-8
- 2'-Acetylacteoside
Catalog No.:BCN3409
CAS No.:94492-24-7
- Tamibarotene
Catalog No.:BCC1983
CAS No.:94497-51-5
- CYM 5541
Catalog No.:BCC6321
CAS No.:945128-26-7
- 7-Prenyljacareubin
Catalog No.:BCN7353
CAS No.:94513-60-7
- 4-Hydroxy-2-methoxyphenol 1-O-(6-O-syringoyl)glucoside
Catalog No.:BCN1300
CAS No.:945259-61-0
- 3,4-Dihydro-6,7-(methylenedioxy)-2(1H)-quinolinone
Catalog No.:BCN1299
CAS No.:94527-34-1
- 20-Deoxocarnosol
Catalog No.:BCN3152
CAS No.:94529-97-2
- Senkyunolide E
Catalog No.:BCC9142
CAS No.:94530-83-3
- Senkyunolide G
Catalog No.:BCC9143
CAS No.:94530-85-5
- 9(11),12-Oleanadien-3-ol
Catalog No.:BCN4499
CAS No.:94530-87-7
- Levcromakalim
Catalog No.:BCC7039
CAS No.:94535-50-9
VX-509 (Decernotinib)-Mediated CYP3A Time-Dependent Inhibition: An Aldehyde Oxidase Metabolite as a Perpetrator of Drug-Drug Interactions.[Pubmed:27298338]
Drug Metab Dispos. 2016 Aug;44(8):1286-95.
(R)-2-((2-(1H-pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)-2-methyl-N-(2,2,2- trifluoroethyl)butanamide (VX-509, decernotinib) is an oral Janus kinase 3 inhibitor that has been studied in patients with rheumatoid arthritis. Patients with rheumatoid arthritis often receive multiple medications, such as statins and steroids, to manage the signs and symptoms of comorbidities, which increases the chances of drug-drug interactions (DDIs). Mechanism-based inhibition is a subset of time-dependent inhibition (TDI) and occurs when a molecule forms a reactive metabolite which irreversibly binds and inactivates drug-metabolizing enzymes, potentially increasing the systemic load to toxic concentrations. Traditionally, perpetrating compounds are screened using human liver microsomes (HLMs); however, this system may be inadequate when the precipitant is activated by a non-cytochrome P450 (P450)-mediated pathway. Even though studies assessing competitive inhibition and TDI using HLM suggested a low risk for CYP3A4-mediated DDI in the clinic, VX-509 increased the area under the curve of midazolam, atorvastatin, and methyl-prednisolone by approximately 12.0-, 2.7-, and 4.3-fold, respectively. Metabolite identification studies using human liver cytosol indicated that VX-509 is converted to an oxidative metabolite, which is the perpetrator of the DDIs observed in the clinic. As opposed to HLM, hepatocytes contain the full complement of drug-metabolizing enzymes and transporters and can be used to assess TDI arising from non-P450-mediated metabolic pathways. In the current study, we highlight the role of aldehyde oxidase in the formation of the hydroxyl-metabolite of VX-509, which is involved in clinically significant TDI-based DDIs and represents an additional example in which a system-dependent prediction of TDI would be evident.
VX-509 (Decernotinib), an Oral Selective JAK-3 Inhibitor, in Combination With Methotrexate in Patients With Rheumatoid Arthritis.[Pubmed:26473751]
Arthritis Rheumatol. 2016 Jan;68(1):46-55.
OBJECTIVE: To assess the efficacy and safety of decernotinib (VX-509), an oral selective inhibitor of JAK-3, in patients with rheumatoid arthritis (RA) in whom the response to methotrexate treatment was inadequate. METHODS: In this 24-week, double-blind, randomized phase IIb study, 358 patients with active RA received either placebo (n = 71) or VX-509 at dosages of 100 mg/day (n = 71), 150 mg/day (n = 72), 200 mg/day (n = 72), or 100 mg twice daily (n = 72). Primary measures of efficacy at week 12 were the response rate according to the American College of Rheumatology 20% improvement criteria (ACR20) and change from baseline in the Disease Activity Score in 28 joints using the C-reactive protein level (DAS28-CRP). RESULTS: At week 12, the ACR20 response rates were 46.5%, 66.7%, 56.9%, and 68.1% in the groups receiving VX-509 at dosages of 100 mg/day, 150 mg/day, 200 mg/day, and 100 mg twice daily, respectively, and 18.3% in the placebo group (P < 0.001 for all comparisons). At week 12, the mean change from baseline in the DAS28-CRP was significantly greater in each VX-509 group compared with the placebo group (P < 0.001). Improvements were maintained at week 24, as shown by the ACR20, ACR50, and ACR70 response rates and mean change from baseline in the DAS28-CRP. The most common adverse event in the VX-509 group was headache (8.7%), and elevated levels of transaminases, lipoproteins, and creatinine were observed. CONCLUSION: VX-509 significantly improved the signs and symptoms of RA at weeks 12 and 24 compared with the placebo group when it was administered in combination with methotrexate. Safety signals included infection and increases in liver transaminase and lipid levels.
Discovery of VX-509 (Decernotinib): A Potent and Selective Janus Kinase 3 Inhibitor for the Treatment of Autoimmune Diseases.[Pubmed:26230873]
J Med Chem. 2015 Sep 24;58(18):7195-216.
While several therapeutic options exist, the need for more effective, safe, and convenient treatment for a variety of autoimmune diseases persists. Targeting the Janus tyrosine kinases (JAKs), which play essential roles in cell signaling responses and can contribute to aberrant immune function associated with disease, has emerged as a novel and attractive approach for the development of new autoimmune disease therapies. We screened our compound library against JAK3, a key signaling kinase in immune cells, and identified multiple scaffolds showing good inhibitory activity for this kinase. A particular scaffold of interest, the 1H-pyrrolo[2,3-b]pyridine series (7-azaindoles), was selected for further optimization in part on the basis of binding affinity (Ki) as well as on the basis of cellular potency. Optimization of this chemical series led to the identification of VX-509 (decernotinib), a novel, potent, and selective JAK3 inhibitor, which demonstrates good efficacy in vivo in the rat host versus graft model (HvG). On the basis of these findings, it appears that VX-509 offers potential for the treatment of a variety of autoimmune diseases.
Efficacy of VX-509 (decernotinib) in combination with a disease-modifying antirheumatic drug in patients with rheumatoid arthritis: clinical and MRI findings.[Pubmed:27084959]
Ann Rheum Dis. 2016 Nov;75(11):1979-1983.
OBJECTIVE: To assess early effects on joint structures of VX-509 in combination with stable disease-modifying antirheumatic drug (DMARD) therapy using MRI in adults with rheumatoid arthritis (RA). METHODS: This phase II, placebo-controlled, double-blind, dose-ranging study randomised patients with RA and inadequate DMARD response to VX-509 100 mg (n=11), 200 mg (n=10) or 300 mg (n=10) or placebo (n=12) once daily for 12 weeks. Outcome measures included American College of Rheumatology score (ACR20; improvement of >/=20%) and disease activity score (DAS28) using C reactive protein (CRP), and the RA MRI scoring (RAMRIS) system. RESULTS: ACR20 response at week 12 was 63.6%, 60.0% and 60.0% in the VX-509 100-mg, 200-mg and 300-mg groups, respectively, compared with 25.0% in the placebo group. DAS28-CRP scores decreased in a dose-dependent manner with increasing VX-509 doses. Decreases in RAMRIS synovitis scores were significantly different from placebo for all VX-509 doses (p<0.01) and for RAMRIS osteitis scores (p<0.01) for VX-509 300 mg. Treatment was generally well tolerated. CONCLUSIONS: VX-509 plus a DMARD reduced the signs and symptoms of RA in patients with an inadequate response to a DMARD alone. MRI responses were detected at week 12. Treatment was generally well tolerated. TRIAL REGISTRATION NUMBER: NCT01754935; results.


