Angiotensin IIPotent vasopressor and a powerful stimulus for production and release of aldosterone from the adrenal cortex. CAS# 68521-88-0 |

- Angiotensin III (human, mouse)
Catalog No.:BCC1031
CAS No.:13602-53-4
- Angiotensin 1/2 (1-9)
Catalog No.:BCC1005
CAS No.:34273-12-6
- Angiotensin I (human, mouse, rat)
Catalog No.:BCC1004
CAS No.:484-42-4
- Angiotensin 1/2 (1-5)
Catalog No.:BCC1035
CAS No.:58442-64-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 68521-88-0 | SDF | Download SDF |
| PubChem ID | 172197 | Appearance | Powder |
| Formula | C50H71N13O12 | M.Wt | 1046.2 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Angiotensin II acetate; Human angiotensin II acetate salt; Angiotensin II acetate human | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Sequence | Asp-Arg-Val-Tyr-Ile-His-Pro-Phe | ||
| Chemical Name | acetic acid;(3S)-3-amino-4-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[(2S)-2-[[(1S)-1-carboxy-2-phenylethyl]carbamoyl]pyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-3-(4-hydroxyphenyl)-1-oxopropan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]amino]-4-oxobutanoic acid | ||
| SMILES | CCC(C)C(C(=O)NC(CC1=CN=CN1)C(=O)N2CCCC2C(=O)NC(CC3=CC=CC=C3)C(=O)O)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(C(C)C)NC(=O)C(CCCN=C(N)N)NC(=O)C(CC(=O)O)N.CC(=O)O | ||
| Standard InChIKey | VBTZKFAHKJXHBA-PIONDTTLSA-N | ||
| Standard InChI | InChI=1S/C50H71N13O12.C2H4O2/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66;1-2(3)4/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55);1H3,(H,3,4)/t28-,33-,34-,35-,36-,37-,38-,40-,41-;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Cell experiment: [1] | |
| Cell lines | Vascular smooth muscle cells |
| Preparation method | The solubility of this peptide in sterile water is >10 mM. Stock solution should be splited and stored at -80°C for several months. |
| Reacting condition | 100 nM, 4 hours |
| Applications | Treatment of Angiotensin II caused a large increase in both NADH and NADPH oxidase activity, both in terms of initial rate and peak response. The increase in oxidase activity was not apparent until ~ 1 hour and continued to increase for at least 6 hours. |
| Animal experiment: [2] | |
| Animal models | C57BL/6J (apoE–/– ) mice |
| Dosage form | Drugs were delivered through the minipumps placed into the subcutaneous space in the back of the neck. 500 or 1000 ng/min/kg for 28 days. |
| Application | Ang II infusion promotes the development of abdominal aortic aneurysms. The region in the abdominal aorta from an Ang II–infused mouse was markedly increased in size. The tissue encompassing this region was resistant to the dissection process typically used to remove adventitial tissue. The bulbous aortic abdominal shape occurred in 20% and 33% of mice in the groups infused with 500 and 1,000 ng/min/kg of Ang II, respectively. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Griendling K K, Minieri C A, Ollerenshaw J D, et al. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circulation research, 1994, 74(6): 1141-1148. [2] Daugherty A, Manning M W, Cassis L A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E–deficient mice. Journal of Clinical Investigation, 2000, 105(11): 1605-1612. | |

Angiotensin II Dilution Calculator

Angiotensin II Molarity Calculator

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Angiotensin II (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe | His-Leu) is an octapeptide that is converted from Angiotensin I (AI) through removal of two C-terminal residues by the enzyme angiotensin-converting enzyme (ACE), primarily through ACE within the lung (but also present in endothelial and kidney epithelial cells).
Angiotensin II has been shown to play important roles in mediating hypertension, heart failure, cardiac remodeling, diabetes, and the proliferative and inflammatory responses to arterial injury [1].
Angiotensin II exerts a wide range of effects on the cardiovascular system. It is a potent vasopressor and a powerful stimulus for production and release of aldosterone from the adrenal cortex. It acts via specific receptors in the adrenal glands to stimulate the secretion of aldosterone, which stimulates salt and water reabsorption by the kidneys. Angiotensin increases blood pressure by stimulating the Gq protein in vascular smooth muscle cells (which in turn activates contraction by an IP3-dependent mechanism). It was demonstrated that Ang II activated phospholipase C, resulting in the production of inositol trisphosphate (IP3) and diacylglycerol, which in turn were responsible for the mobilization of [Ca2+]i and the activation of PKC, respectively. Additional studies in SMC cultures examined the properties of Ang II as a growth agonist, in particular its role in promoting cellular hypertrophy, characterized by increases in protein synthesis, cell size, and polyploidy. [2][3]
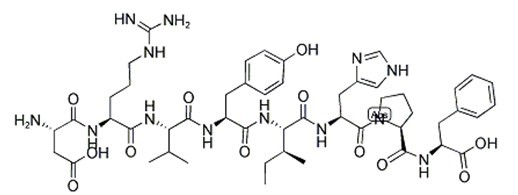
Fig. 2: Formula of Angiotensin II
Fig. 2: Structure of Angiotensin II
Ref:
1. Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Suzuki Y, Mezzano S, Plaza JJ, Egido J. Role of the renin-angiotensin system in vascular diseases: expanding the field. Hypertension. 2001; 38: 1382–1387.
2. Geisterfer AA, Peach MJ, Owens GK. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988; 62: 749–756.
3. Berk BC, Vekshtein V, Gordon HM, Tsuda T. Angiotensin II-stimulated protein synthesis in cultured vascular smooth muscle cells. Hypertension. 1989; 13: 305–314.
- Vigabatrin
Catalog No.:BCC2039
CAS No.:68506-86-5
- Pramiracetam
Catalog No.:BCC4928
CAS No.:68497-62-1
- WS 12
Catalog No.:BCC7543
CAS No.:68489-09-8
- Coromandaline
Catalog No.:BCN2044
CAS No.:68473-86-9
- Heliovicine
Catalog No.:BCN2047
CAS No.:68473-85-8
- 4-(p-Biphenylyl)-3-hydroxybutyric acid
Catalog No.:BCN2240
CAS No.:6845-17-6
- Isowighteone
Catalog No.:BCN4243
CAS No.:68436-47-5
- PPDA
Catalog No.:BCC5918
CAS No.:684283-16-7
- Timosaponin AI
Catalog No.:BCN7819
CAS No.:68422-00-4
- 6',7'-Dihydroxybergamottin acetonide
Catalog No.:BCN4242
CAS No.:684217-08-1
- 20-O-Glucoginsenoside Rf
Catalog No.:BCN8220
CAS No.:68406-27-9
- Ginsenoside Rb3
Catalog No.:BCN1065
CAS No.:68406-26-8
- Isoguvacine hydrochloride
Catalog No.:BCC6575
CAS No.:68547-97-7
- Cilostamide
Catalog No.:BCC6843
CAS No.:68550-75-4
- GSK 264220A
Catalog No.:BCC6062
CAS No.:685506-42-7
- Eupatoriopicrin
Catalog No.:BCN7116
CAS No.:6856-01-5
- Pridinol Methanesulfonate
Catalog No.:BCC3845
CAS No.:6856-31-1
- Moschamine
Catalog No.:BCN3900
CAS No.:68573-23-9
- Prometaphanine
Catalog No.:BCN4244
CAS No.:6858-85-1
- PX-478 2HCl
Catalog No.:BCC6502
CAS No.:685898-44-6
- Isorhyncophylline
Catalog No.:BCN3466
CAS No.:6859-01-4
- Isorhynchophylline
Catalog No.:BCN6458
CAS No.:6859-1-4
- Xylobiose
Catalog No.:BCN8424
CAS No.:6860-47-5
- Procerine
Catalog No.:BCN2017
CAS No.:68622-81-1
Angiotensin II Peptide Vaccine Protects Ischemic Brain Through Reducing Oxidative Stress.[Pubmed:28364024]
Stroke. 2017 May;48(5):1362-1368.
BACKGROUND AND PURPOSE: Medication nonadherence is one of major risk factors for the poor outcome in ischemic stroke. Vaccination is expected to solve such a problem because of its long-lasting effects, but its effect on ischemic brain damage is still unknown. Here, we focused on vaccination for renin-angiotensin system and examined the effects of Angiotensin II (Ang II) peptide vaccine in permanent middle cerebral artery occlusion model in rats. METHODS: Male Wistar rats were exposed to permanent middle cerebral artery occlusion after 3x injections of Ang II peptide vaccine, and the serum or brain level of anti-Ang II antibody was examined. The effects of the vaccine were evaluated by differences in infarction volume, brain renin-angiotensin system components, and markers for neurodegeneration and oxidative stress. RESULTS: Ang II vaccination successfully produced anti-Ang II antibodies in serum without concomitant change in blood pressure. Sufficient production of serum anti-Ang II antibody led to reduction of infarct volume and induced the penetration of anti-Ang II antibody in ischemic hemisphere, with suppressed expression of Ang II type 1 receptor mRNA. Vaccinated rats with sufficient antibody production showed the reduction of Fluoro-Jade B-positive cells, spectrin fragmentation, 4-hydroxynonenal-positive cells, and Nox 2 mRNA expression. CONCLUSIONS: Our findings indicate that Ang II vaccination exerts neuroprotective and antioxidative effects in cerebral ischemia, with renin-angiotensin system blockade by penetration of anti-Ang II antibodies into ischemic brain lesion. Ang II peptide vaccination could be a promising approach to treat ischemic stroke.
Potential of angiotensin II receptor blockers in the treatment of diabetic retinopathy.[Pubmed:28363842]
Life Sci. 2017 May 1;176:1-9.
Impairments in renin-angiotensin-aldosterone system during diabetes mellitus, leading to its dysfunction, have been known since a decade. Hyperglycemia induces several pathological alterations which upregulate this system, accounting for overexpression of its downstream signaling molecules, amongst which Angiotensin II (Ang II) and aldosterone hold prime pathological notoriety. Although it is well known that elevated plasma levels of Ang II play a crucial role in the pathophysiology of type-II diabetic mellitus, by inducing and aggravating insulin resistance, it is not quite much known that it equally elevates the possibility/risk of development of diabetic complications. Also, amongst the various diabetic complications, the effects of Ang II upregulation are more widely acknowledged in studies concerning the pathophysiology of diabetic nephropathy; however, its role in exacerbating diabetic retinopathy has not received much attention. Ang II, indeed, plays a detrimental role in the progression of diabetic retinopathy by augmenting the principal events involved in its pathogenesis, namely oxidative stress, angiogenesis and inflammation. The utility of angiotensin receptor blockers has shown positive results in research studies and hence, might potentially provide a novel adjuvant therapy for treating this complication and preventing the associated vision-loss in diabetic patients.
ELABELA-APJ axis protects from pressure overload heart failure and angiotensin II-induced cardiac damage.[Pubmed:28371822]
Cardiovasc Res. 2017 Jun 1;113(7):760-769.
Aims: Elabela/Toddler/Apela (ELA) has been identified as a novel endogenous peptide ligand for APJ/Apelin receptor/Aplnr. ELA plays a crucial role in early cardiac development of zebrafish as well as in maintenance of self-renewal of human embryonic stem cells. Apelin was the first identified APJ ligand, and exerts positive inotropic heart effects and regulates the renin-angiotensin system. The aim of this study was to investigate the biological effects of ELA in the cardiovascular system. Methods and results: Continuous infusion of ELA peptide significantly suppressed pressure overload-induced cardiac hypertrophy, fibrosis and impaired contractility in mice. ELA treatment reduced mRNA expression levels of genes associated with heart failure and fibrosis. The cardioprotective effects of ELA were diminished in APJ knockout mice, indicating that APJ is the key receptor for ELA in the adult heart. Mechanistically, ELA downregulated angiotensin-converting enzyme (ACE) expression in the stressed hearts, whereas it showed little effects on angiotensin-converting enzyme 2 (ACE2) expression, which are distinct from the effects of Apelin. FoxM1 transcription factor, which induces ACE expression in the stressed hearts, was downregulated by ELA but not by Apelin. ELA antagonized Angiotensin II-induced hypertension, cardiac hypertrophy, and fibrosis in mice. Conclusion: The ELA-APJ axis protects from pressure overload-induced heart failure possibly via suppression of ACE expression and pathogenic Angiotensin II signalling. The different effects of ELA and Apelin on the expression of ACE and ACE2 implicate fine-tuned mechanisms for a ligand-induced APJ activation and downstream signalling.
High serum thrombospondin-1 concentration is associated with slower abdominal aortic aneurysm growth and deficiency of thrombospondin-1 promotes angiotensin II induced aortic aneurysm in mice.[Pubmed:28364044]
Clin Sci (Lond). 2017 Jun 7;131(12):1261-1281.
Abdominal aortic aneurysm (AAA) is a common age-related vascular disease characterized by progressive weakening and dilatation of the aortic wall. Thrombospondin-1 (TSP-1; gene Thbs1) is a member of the matricellular protein family important in the control of extracellular matrix (ECM) remodelling. In the present study, the association of serum TSP-1 concentration with AAA progression was assessed in 276 men that underwent repeated ultrasound for a median 5.5 years. AAA growth was negatively correlated with serum TSP-1 concentration (Spearman's rho (-)0.129, P=0.033). Men with TSP-1 in the highest quartile had a reduced likelihood of AAA growth greater than median during follow-up (OR: 0.40; 95% confidence interval (CI): 0.19-0.84, P=0.016, adjusted for other risk factors). Immunohistochemical staining for TSP-1 was reduced in AAA body tissues compared with the relatively normal AAA neck. To further assess the role of TSP-1 in AAA initiation and progression, combined TSP-1 and apolipoprotein deficient (Thbs1(-/-)ApoE(-/-), n=20) and control mice (ApoE(-/-), n=20) were infused subcutaneously with Angiotensin II (AngII) for 28 days. Following AngII infusion, Thbs1(-/-) ApoE(-/-) mice had larger AAAs by ultrasound (P=0.024) and ex vivo morphometry measurement (P=0.006). The Thbs1(-/-)ApoE(-/-) mice also showed increased elastin filament degradation along with elevated systemic levels and aortic expression of matrix metalloproteinase (MMP)-9. Suprarenal aortic segments and vascular smooth muscle cells (VSMCs) isolated from Thbs1(-/-)ApoE(-/-) mice showed reduced collagen 3A1 gene expression. Furthermore, Thbs1(-/-)ApoE(-/-) mice had reduced aortic expression of low-density lipoprotein (LDL) receptor-related protein 1. Collectively, findings from the present study suggest that TSP-1 deficiency promotes maladaptive remodelling of the ECM leading to accelerated AAA progression.


