C-1Protein kinase C inhibitor CAS# 84468-24-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84468-24-6 | SDF | Download SDF |
| PubChem ID | 3545 | Appearance | Powder |
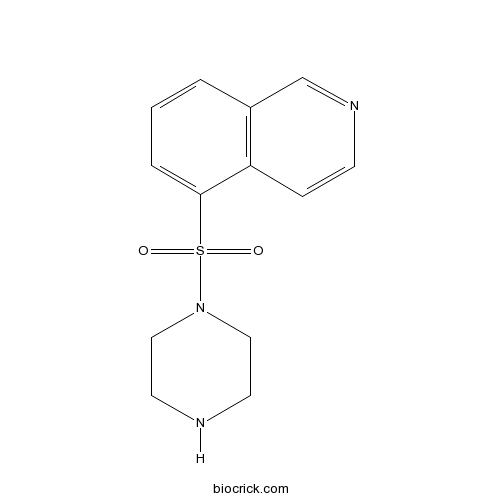
| Formula | C13H15N3O2S | M.Wt | 277.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 150 mg/mL (540.85 mM; Need ultrasonic and warming) | ||
| Chemical Name | 5-piperazin-1-ylsulfonylisoquinoline | ||
| SMILES | C1CN(CCN1)S(=O)(=O)C2=CC=CC3=C2C=CN=C3 | ||
| Standard InChIKey | UPTYCYWTFGTCCG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H15N3O2S/c17-19(18,16-8-6-14-7-9-16)13-3-1-2-11-10-15-5-4-12(11)13/h1-5,10,14H,6-9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of protein kinase C. Vasodilator. |

C-1 Dilution Calculator

C-1 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6057 mL | 18.0284 mL | 36.0568 mL | 72.1137 mL | 90.1421 mL |
| 5 mM | 0.7211 mL | 3.6057 mL | 7.2114 mL | 14.4227 mL | 18.0284 mL |
| 10 mM | 0.3606 mL | 1.8028 mL | 3.6057 mL | 7.2114 mL | 9.0142 mL |
| 50 mM | 0.0721 mL | 0.3606 mL | 0.7211 mL | 1.4423 mL | 1.8028 mL |
| 100 mM | 0.0361 mL | 0.1803 mL | 0.3606 mL | 0.7211 mL | 0.9014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- threo-Guaiacylglycerol-beta-O-4'-dehydrodisinapyl ether
Catalog No.:BCN6928
CAS No.:844637-85-0
- β-CCB
Catalog No.:BCC6635
CAS No.:84454-35-3
- A-769662
Catalog No.:BCC2080
CAS No.:844499-71-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
- Fmoc-NH2
Catalog No.:BCC2803
CAS No.:84418-43-9
- 23-Hydroxybetulin
Catalog No.:BCN6463
CAS No.:84414-40-4
- alpha-Arbutin
Catalog No.:BCN8336
CAS No.:84380-01-8
- Bretazenil
Catalog No.:BCC7711
CAS No.:84379-13-5
- Neuromedin S (rat)
Catalog No.:BCC6055
CAS No.:843782-19-4
- Adoxosidic acid
Catalog No.:BCN7593
CAS No.:84375-46-2
- Mifepristone
Catalog No.:BCC4486
CAS No.:84371-65-3
- Bedaquiline
Catalog No.:BCC5246
CAS No.:843663-66-1
- BTS 54-505 hydrochloride
Catalog No.:BCC5901
CAS No.:84484-78-6
- Sibutramine hydrochloride
Catalog No.:BCC5252
CAS No.:84485-00-7
- Varlitinib (ARRY334543)
Catalog No.:BCC3725
CAS No.:845272-21-1
- Sevelamer Carbonate
Catalog No.:BCC4717
CAS No.:845273-93-0
- ICI 162,846
Catalog No.:BCC6808
CAS No.:84545-30-2
- Bedaquiline fumarate
Catalog No.:BCC5245
CAS No.:845533-86-0
- MNI-caged-D-aspartate
Catalog No.:BCC5896
CAS No.:845555-94-4
- Bitopertin
Catalog No.:BCC1419
CAS No.:845614-11-1
- Bitopertin (R enantiomer)
Catalog No.:BCC1420
CAS No.:845614-12-2
- 4-Hydroxycephalotaxine
Catalog No.:BCN4386
CAS No.:84567-08-8
- PHA-767491
Catalog No.:BCC1858
CAS No.:845714-00-3
- Rocaglamide
Catalog No.:BCN4387
CAS No.:84573-16-0
Design, synthesis and evaluation of 4-substituted anthra[2,1-c][1,2,5]thiadiazole-6,11-dione derivatives as novel non-camptothecin topoisomerase I inhibitors.[Pubmed:28351590]
Bioorg Med Chem Lett. 2017 May 1;27(9):1929-1933.
Previously, 4-tosylanthra[1,2-c][1,2,5]thiadiazole-6,11-dione (1) was identified as a novel non-camptothecin topoisomerase I (Top1) inhibitor by structure-based virtual screening. Herein, a series of 4-substituted derivatives were designed and synthesized. Most of them showed potent Top1 inhibitory activity. Their in vitro antiproliferative activity was also evaluated in A549, HCT-116 and ZR-75-30 human cancer cell lines. Compound 8s showed good antiproliferative activity with IC50 of 0.52muM and 0.42muM against HCT-116 and ZR-75-30 cell line, respectively. Top1 unwinding assay and molecular modeling studies rationalized the mode of action of this new class of inhibitors.
XEN Glaucoma Implant with Mitomycin C 1-Year Follow-Up: Result and Complications.[Pubmed:28348884]
J Ophthalmol. 2017;2017:5457246.
Purpose. To evaluate gel microstent (XEN, Aquesys, Inc) for treatment of primary open angle glaucoma (POAG). Methods. In this prospective interventional study, 13 eyes with POAG underwent XEN implantation with subconjunctival mitomycin-C. Of those eyes, 3 were pseudophakic and 10 underwent simultaneous phacoemulsification and XEN. Patients had uncontrolled IOP, had intolerance to therapy, or had maximal therapy but undergoing cataract extraction. Follow-up visits included IOP, number of medications, vision, and complications and lasted for 1 year. Complete success was defined as IOP reduction >/=20% from preoperative baseline at 1 year without any glaucoma medications while partial success as IOP reduction of >/=20% at 1 year with medications. Results. IOP dropped from 16 +/- 4 mmHg pre-op to 9 +/- 5, 11 +/- 6, 12 +/- 5, 12 +/- 4, and 12 +/- 3 mmHg at 1 week, 1, 3, 6, and 12 months (p = 0.004, 0.026, 0.034, 0.01, and 0.01, Wilcoxon Signed Ranks) consecutively. BCVA (LogMAR) was 0.33 +/- 0.34 and improved to 0.13 +/- 0.11 at 1 year. Mean number of medications dropped from 1.9 +/- 1 preoperatively to 0.3 +/- 0.49 (p = 0.003) at 1 year. 42% of eyes achieved complete success and 66% qualified success. Complications included choroidal detachment in 2 eyes, and implant extrusion in 1 eye, and 2 eyes underwent trabeculectomy. Conclusion. XEN implant is an effective surgical treatment for POAG, with significant reduction in IOP and glaucoma medications at 1 year follow-up.
Posterior arch C-1 screw technique: a cadaveric comparison study.[Pubmed:28304240]
J Neurosurg Spine. 2017 Jun;26(6):679-683.
OBJECTIVE Posterior atlantoaxial stabilization and fusion using C-1 lateral mass screw fixation has become commonly used in the treatment of instability and for reconstructive indications since its introduction by Goel and Laheri in 1994 and modification by Harms in 2001. Placement of such lateral mass screws can be challenging because of the proximity to the spinal cord, vertebral artery, an extensive venous plexus, and the C-2 nerve root, which overlies the designated starting point on the posterior center of the lateral mass. An alternative posterior access point starting on the posterior arch of C-1 could provide a C-2 nerve root-sparing starting point for screw placement, with the potential benefit of greater directional control and simpler trajectory. The authors present a cadaveric study comparing an alternative strategy (i.e., a C-1 screw with a posterior arch starting point) to the conventional strategy (i.e., using the lower lateral mass entry site), specifically assessing the safety of screw placement to preserve the C-2 nerve root. METHODS Five US-trained spine fellows instrumented 17 fresh human cadaveric heads using the Goel/Harms C-1 lateral mass (GHLM) technique on the left and the posterior arch lateral mass (PALM) technique on the right, under fluoroscopic guidance. After screw placement, a CT scan was obtained on each specimen to assess for radiographic screw placement accuracy. Four faculty spine surgeons, blinded to the surgeon who instrumented the cadaver, independently graded the quality of screw placement using a modified Upendra classification. RESULTS Of the 17 specimens, the C-2 nerve root was anatomically impinged in 13 (76.5%) of the specimens. The GHLM technique was graded Type 1 or 2, which is considered "acceptable," in 12 specimens (70.6%), and graded Type 3 or 4 ("unacceptable") in 5 specimens (29.4%). In contrast, the PALM technique had 17 (100%) of 17 graded Type 1 or 2 (p = 0.015). There were no vertebral artery injuries found in either technique. All screw violations occurred in the medial direction. CONCLUSIONS The PALM technique showed statistically fewer medial penetrations than the GHLM technique in this study. The reason for this is not clear, but may stem from a more angulated "up-and-in" screw direction necessary with a lower starting point.
Crystal structure of 5,6-bis(9H-carbazol-9-yl)benzo[c][1,2,5]thiadiazole: distortion from a hypothetical higher-symmetry structure.[Pubmed:28378715]
Acta Crystallogr C Struct Chem. 2017 Apr 1;73(Pt 4):319-324.
Nucleophilic substitution of F atoms in 5,6-difluorobenzo[c][1,2,5]thiadiazole (DFBT) for carbazole could be potentially interesting as a novel way of synthesizing building blocks for new conjugated materials for applications in organic chemistry. The crystal structures of 5,6-bis(9H-carbazol-9-yl)benzo[c][1,2,5]thiadiazole (DCBT), C30H18N4S, and its hydrate, C30H18N4S.0.125H2O, were investigated using single-crystal X-ray analysis. The hydrate contains two symmetry-independent DCBT molecules. The dihedral angles between the plane of the central benzothiadiazole fragment and that of the carbazole units vary between 50.8 and 69.9 degrees , indicating conformational flexibility of the DCBT molecule in the crystals, which is consistent with quantum chemical calculations. The analysis of the crystal packing of DCBT revealed that the experimental triclinic structure could be described as a distortion from a hypothetical higher-symmetry monoclinic structure. The quantum chemical calculations of two possible monoclinic structures, which are related to the experimental structure by a shifting of molecular layers, showed that the proposed structures are higher in energy by 5.4 and 10.1 kcal mol(-1). This energy increase is caused by less dense crystal packings of the symmetric structures, which results in a decrease of the number of intermolecular interactions.
Role of protein kinases in stimulation of human polymorphonuclear leukocyte oxidative metabolism by various agonists. Differential effects of a novel protein kinase inhibitor.[Pubmed:3003155]
J Clin Invest. 1986 Jan;77(1):61-5.
Isoquinoline sulfonamides have recently been shown to exert novel inhibitory effects on mammalian protein kinases by competitively binding to the ATP substrate site (Hidaka, H., M. Inagaki, S. Kawamoto, and Y. Sasaki, 1984, Biochemistry, 23: 5036-5041). We synthesized a unique analog of the previously reported compounds, 1-(5-isoquinolinesulfonyl) piperazine (C-I), in order to assess the role of protein kinases in modulating the agonist-stimulated oxidative burst of human polymorphonuclear leukocytes (PMN). Compound C-I, at micromolar concentration, markedly inhibited the release of superoxide anion from human PMN stimulated with phorbol myristate acetate or the synthetic diacylglycerol, 1-oleoyl-2-acetyl glycerol. These data are consonant with previously reported data which indicate that the calcium and phospholipid-dependent protein kinase, protein kinase C, serves as the intracellular receptor for these agonists. In contrast, superoxide anion production stimulated by the complement anaphylatoxin peptide C5a or the synthetic chemotaxin formyl-methionyl-leucyl-phenylalanine were not inhibited by C-I. These data suggest that parallel pathways exist for the agonist-stimulated respiratory burst of human neutrophils, only one of which utilizes the calcium and phospholipid-dependent protein kinase.


