β-CCBBenzodiazepine inverse agonist, putative endogenous ligand CAS# 84454-35-3 |
- Bestatin trifluoroacetate
Catalog No.:BCC3909
CAS No.:223763-80-2
- Fumagillin
Catalog No.:BCC2347
CAS No.:23110-15-8
- Tosedostat (CHR2797)
Catalog No.:BCC2309
CAS No.:238750-77-1
- Bestatin hydrochloride
Catalog No.:BCC3908
CAS No.:65391-42-6
- Alogliptin (SYR-322)
Catalog No.:BCC2113
CAS No.:850649-61-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 84454-35-3 | SDF | Download SDF |
| PubChem ID | 128618 | Appearance | Powder |
| Formula | C16H16N2O2 | M.Wt | 268.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 5 mM in ethanol | ||
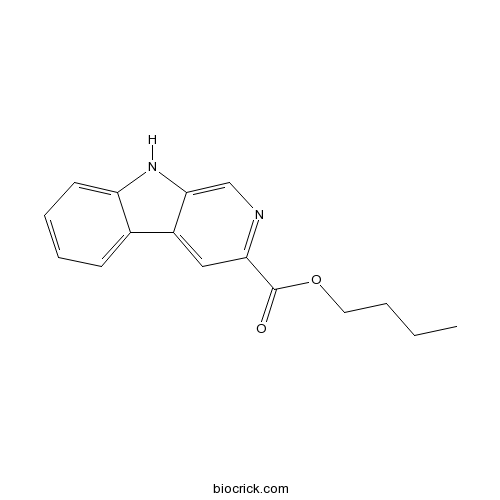
| Chemical Name | butyl 9H-pyrido[3,4-b]indole-3-carboxylate | ||
| SMILES | CCCCOC(=O)C1=NC=C2C(=C1)C3=CC=CC=C3N2 | ||
| Standard InChIKey | WGNGIELOOKACSB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16N2O2/c1-2-3-8-20-16(19)14-9-12-11-6-4-5-7-13(11)18-15(12)10-17-14/h4-7,9-10,18H,2-3,8H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | An endogenous proconvulsant and anxiogenic benzodiazepine receptor ligand. |

β-CCB Dilution Calculator

β-CCB Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.727 mL | 18.6352 mL | 37.2703 mL | 74.5406 mL | 93.1758 mL |
| 5 mM | 0.7454 mL | 3.727 mL | 7.4541 mL | 14.9081 mL | 18.6352 mL |
| 10 mM | 0.3727 mL | 1.8635 mL | 3.727 mL | 7.4541 mL | 9.3176 mL |
| 50 mM | 0.0745 mL | 0.3727 mL | 0.7454 mL | 1.4908 mL | 1.8635 mL |
| 100 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- A-769662
Catalog No.:BCC2080
CAS No.:844499-71-4
- AT7519
Catalog No.:BCC2541
CAS No.:844442-38-2
- Fmoc-NH2
Catalog No.:BCC2803
CAS No.:84418-43-9
- 23-Hydroxybetulin
Catalog No.:BCN6463
CAS No.:84414-40-4
- alpha-Arbutin
Catalog No.:BCN8336
CAS No.:84380-01-8
- Bretazenil
Catalog No.:BCC7711
CAS No.:84379-13-5
- Neuromedin S (rat)
Catalog No.:BCC6055
CAS No.:843782-19-4
- Adoxosidic acid
Catalog No.:BCN7593
CAS No.:84375-46-2
- Mifepristone
Catalog No.:BCC4486
CAS No.:84371-65-3
- Bedaquiline
Catalog No.:BCC5246
CAS No.:843663-66-1
- Rabdosin B
Catalog No.:BCN3236
CAS No.:84304-92-7
- 4,4'-Cyclohexylidenebisphenol
Catalog No.:BCC8663
CAS No.:843-55-0
- threo-Guaiacylglycerol-beta-O-4'-dehydrodisinapyl ether
Catalog No.:BCN6928
CAS No.:844637-85-0
- C-1
Catalog No.:BCC6687
CAS No.:84468-24-6
- BTS 54-505 hydrochloride
Catalog No.:BCC5901
CAS No.:84484-78-6
- Sibutramine hydrochloride
Catalog No.:BCC5252
CAS No.:84485-00-7
- Varlitinib (ARRY334543)
Catalog No.:BCC3725
CAS No.:845272-21-1
- Sevelamer Carbonate
Catalog No.:BCC4717
CAS No.:845273-93-0
- ICI 162,846
Catalog No.:BCC6808
CAS No.:84545-30-2
- Bedaquiline fumarate
Catalog No.:BCC5245
CAS No.:845533-86-0
- MNI-caged-D-aspartate
Catalog No.:BCC5896
CAS No.:845555-94-4
- Bitopertin
Catalog No.:BCC1419
CAS No.:845614-11-1
- Bitopertin (R enantiomer)
Catalog No.:BCC1420
CAS No.:845614-12-2
- 4-Hydroxycephalotaxine
Catalog No.:BCN4386
CAS No.:84567-08-8
Isolation and identification in bovine cerebral cortex of n-butyl beta-carboline-3-carboxylate, a potent benzodiazepine binding inhibitor.[Pubmed:3014522]
Proc Natl Acad Sci U S A. 1986 Jul;83(13):4952-6.
A substance having benzodiazepine-binding inhibitory activity has been extracted from 18 kg of gray matter of bovine cerebral cortex and purified to homogeneity. This substance inhibits competitively [3H]flunitrazepam and ethyl beta-[3H]carboline-3-carboxylate binding with high affinity (Ki, 3 nM), but it is inactive upon 3H-labeled Ro 5-4864, [3H]quinuclidinyl benzylate, [3H]prazosin, [3H]clonidine, [3H]dihydroalprenolol, and upon high-affinity [3H]muscimol binding. This inhibitor has been identified as n-butyl beta-carboline-3-carboxylate (beta-CCB) by fast atom bombardment mass spectroscopy (Mr, 268) and electron bombardment fragmentography, ultraviolet and fluorescence spectra, coelution in HPLC with standard beta-CCB, and by the exact correspondence in Ki with beta-CCB on the displacement of [3H]flunitrazepam binding. The possible artificial formation of beta-CCB has been discarded by a series of control experiments including addition of tryptophan to the starting homogenate, extraction from liver, isolation and purification by an alternative procedure avoiding organic solvents, and by the impossibility of making beta-CCB from beta-carboline-3-carboxylic acid or its methyl ester in the conditions of our extraction and purification procedures.


