Cimicifugic acid FCAS# 220618-91-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 220618-91-7 | SDF | Download SDF |
| PubChem ID | 6450179 | Appearance | Light yellow powder |
| Formula | C21H20O10 | M.Wt | 432.4 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
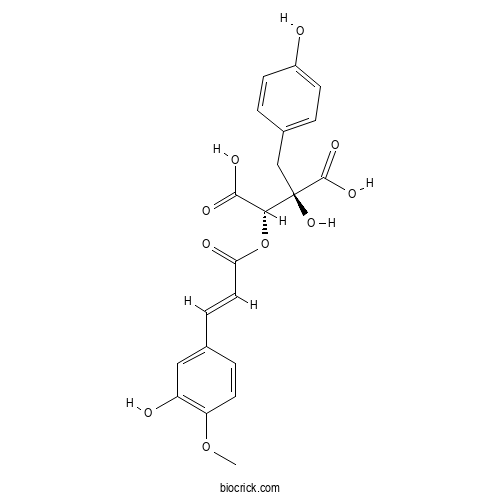
| Chemical Name | (2R,3S)-2-hydroxy-3-[(E)-3-(3-hydroxy-4-methoxyphenyl)prop-2-enoyl]oxy-2-[(4-hydroxyphenyl)methyl]butanedioic acid | ||
| SMILES | COC1=C(C=C(C=C1)C=CC(=O)OC(C(=O)O)C(CC2=CC=C(C=C2)O)(C(=O)O)O)O | ||
| Standard InChIKey | WBGMKAAMRFEBHK-PZTMCFHLSA-N | ||
| Standard InChI | InChI=1S/C21H20O10/c1-30-16-8-4-12(10-15(16)23)5-9-17(24)31-18(19(25)26)21(29,20(27)28)11-13-2-6-14(22)7-3-13/h2-10,18,22-23,29H,11H2,1H3,(H,25,26)(H,27,28)/b9-5+/t18-,21-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. 2. 3. Cimicifugic acid F shows | |||||

Cimicifugic acid F Dilution Calculator

Cimicifugic acid F Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3127 mL | 11.5634 mL | 23.1267 mL | 46.2535 mL | 57.8168 mL |
| 5 mM | 0.4625 mL | 2.3127 mL | 4.6253 mL | 9.2507 mL | 11.5634 mL |
| 10 mM | 0.2313 mL | 1.1563 mL | 2.3127 mL | 4.6253 mL | 5.7817 mL |
| 50 mM | 0.0463 mL | 0.2313 mL | 0.4625 mL | 0.9251 mL | 1.1563 mL |
| 100 mM | 0.0231 mL | 0.1156 mL | 0.2313 mL | 0.4625 mL | 0.5782 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- beta-Citronellol
Catalog No.:BCN9820
CAS No.:106-22-9
- trans-Fertaric acid
Catalog No.:BCN9819
CAS No.:74282-22-7
- Tricetin
Catalog No.:BCN9818
CAS No.:520-31-0
- Withanoside V
Catalog No.:BCN9817
CAS No.:256520-90-8
- 3-Aminocoumarin
Catalog No.:BCN9816
CAS No.:1635-31-0
- 7-Ethoxy-4-methylcoumarin
Catalog No.:BCN9815
CAS No.:87-05-8
- Vicinin 2
Catalog No.:BCN9814
CAS No.:90456-53-4
- 1,2,3-Tri-n-Octanoylglycerol
Catalog No.:BCN9813
CAS No.:538-23-8
- Norcamphor
Catalog No.:BCN9812
CAS No.:497-38-1
- 3-Hydroxycoumarin
Catalog No.:BCN9811
CAS No.:939-19-5
- Quercetin 3-rutinoside 7-glucoside
Catalog No.:BCN9810
CAS No.:30311-61-6
- Isoedultin
Catalog No.:BCN9809
CAS No.:43043-08-9
- Eclalbasaponin II
Catalog No.:BCN9822
CAS No.:78285-90-3
- Morindin
Catalog No.:BCN9823
CAS No.:60450-21-7
- Acetic acid hexyl ester
Catalog No.:BCN9824
CAS No.:142-92-7
- Grayanotoxin I
Catalog No.:BCN9825
CAS No.:4720-09-6
- Vaccarin E
Catalog No.:BCN9826
CAS No.:2252345-81-4
- 6-Hydroxyflavone
Catalog No.:BCN9827
CAS No.:6665-83-4
- Picrotoxinin
Catalog No.:BCN9828
CAS No.:17617-45-7
- 4,4'-Dimethoxychalcone
Catalog No.:BCN9829
CAS No.:2373-89-9
- Urushiol (15:1)
Catalog No.:BCN9830
CAS No.:35237-02-6
- 28-Homobrassinolide
Catalog No.:BCN9831
CAS No.:82373-95-3
- Ethyl caproate
Catalog No.:BCN9832
CAS No.:123-66-0
- Teupolioside
Catalog No.:BCN9833
CAS No.:143617-02-1
Identification of caffeic acid derivatives in Actea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry.[Pubmed:12717772]
Rapid Commun Mass Spectrom. 2003;17(9):978-82.
Caffeic acid derivatives occurring in black cohosh [Cimicifuga racemosa (L.) Nutt., Actaea racemosa (Ranunculaceae)], some of which may have pharmacological activity, were analyzed using high-performance liquid chromatography (HPLC) electrospray ionization tandem mass spectrometry (ESI-MS/MS) with the aim of developing a methodology for their rapid identification in a complex plant matrix. Based on these studies, structurally characteristic product ions and neutral molecule losses were identified, which were then used during LC/MS/MS with product ion scanning, precursor scanning and constant neutral loss scanning to detect caffeic acid derivatives in a crude extract of black cohosh. Several caffeic acid derivatives were detected, and the identification of six of them were confirmed by comparison with authentic standards including caffeic acid, ferulic acid, isoferulic acid, fukinolic acid, cimicifugic acid A, and cimicifugic acid B. Four other compounds were detected that appeared to be caffeic acid derivatives based on LC/MS/MS retention times, molecular weights, and fragmentation patterns during MS/MS. Since standards were unavailable for these four compounds, they were tentatively identified using LC/MS/MS as cimicifugic acid E, Cimicifugic acid F, dehydrocimicifugic acid A, and dehydrocimicifugic acid B. Dehydrocimicifugic acid A and dehydrocimicifugic acid B have not been reported previously to be constituents of black cohosh.
Black cohosh (Cimicifuga racemosa L.) protects against menadione-induced DNA damage through scavenging of reactive oxygen species: bioassay-directed isolation and characterization of active principles.[Pubmed:12428954]
J Agric Food Chem. 2002 Nov 20;50(24):7022-8.
The roots/rhizomes of Cimicifuga racemosa L. (Nutt.) (black cohosh) have traditionally been used to treat menopausal symptoms through an unknown mechanism of action. In an effort to determine if black cohosh had additional health benefits, methanol extracts were investigated for their potential to scavenge reactive oxygen species and to protect against menadione-induced DNA damage. These extracts effectively scavenged 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals. In addition, the extracts showed dose-dependent decreases in DNA single-strand breaks and oxidized bases induced by the quinone menadione using the comet (single-cell gel electrophoresis assay) and fragment length associated repair enzyme assays, respectively. Bioassay-directed fractionation of the methanolic extracts using the DPPH assay as a monitor led to the isolation of nine antioxidant active compounds: caffeic acid (1), methyl caffeate (2), ferulic acid (3), isoferulic acid (4), fukinolic acid (5), cimicifugic acid A (6), cimicifugic acid B (7), Cimicifugic acid F (8), cimiracemate A (9), and cimiracemate B (10). Six of these antioxidants were found to reduce menadione-induced DNA damage in cultured S30 breast cancer cells with the following order of potency: methyl caffeate (2) > caffeic acid (1) > ferulic acid (3) > cimiracemate A (9) > cimiracemate B (10) > fukinolic acid (5). These data suggest that black cohosh can protect against cellular DNA damage caused by reactive oxygen species by acting as antioxidants.
Inhibition of neutrophil elastase activity by cinnamic acid derivatives from Cimicifuga racemosa.[Pubmed:11199135]
Planta Med. 2000 Dec;66(8):751-3.
Caffeic acid, fukinolic acid as well as cimicifugic acids A, B, E and F isolated from the rhizomes of Cimicifuga racemosa (Ranunculaceae) inhibited the activity of neutrophil elastase (EC 3.4.21.37) in a dose-dependent manner. An IC50 of 93 mumol/L was determined for caffeic acid and 0.23 mumol/L for fukinolic acid. Cimicifugic acid A inhibited the enzyme with an IC50 of 2.2 mumol/L, cimicifugic acid B with 11.4 mumol/L, and Cimicifugic acid F with 18 mumol/L. Cimicifugic acid E was only a very weak inhibitor.
Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid.[Pubmed:10630125]
Planta Med. 1999 Dec;65(8):763-4.
Hydroxycinnamic acid esters of fukiic acid and piscidic acid were isolated from a 50% ethanolic extract obtained from the rhizomes of Cimicifuga racemosa (Ranunculaceae). Besides 2-E-caffeoylfukiic acid (fukinolic acid), 2-E-feruloylfukiic acid (cimicifugic acid A), 2-E-isoferuloylfukiic acid (cimicifugic acid B), 2-E-feruloylpiscidic acid (cimicifugic acid E) and 2-E-isoferuloylpiscidic acid (Cimicifugic acid F), free caffeic, ferulic and isoferulic acids were isolated. The estrogenic activity of fukinolic acid was shown by increased proliferation (126% at 5 x 10(-8) M) of an estrogen dependent MCF-7 cell system with reference to estradiol (120% at 10(-10) M).


