TeupoliosideCAS# 143617-02-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 143617-02-1 | SDF | Download SDF |
| PubChem ID | 16062094 | Appearance | Beige powder |
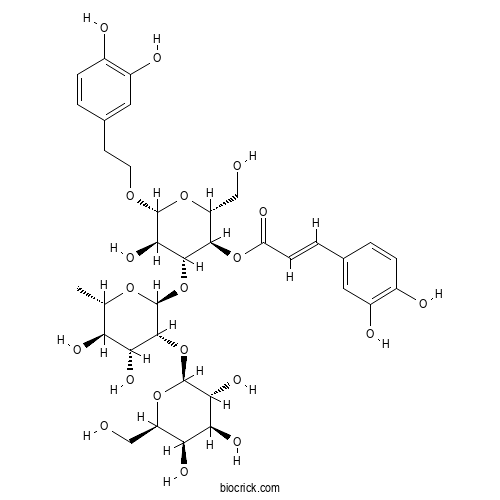
| Formula | C35H46O20 | M.Wt | 786.7 |
| Type of Compound | Polyphenols | Storage | Desiccate at -20°C |
| Synonyms | Lamiuside A; Lamalboside | ||
| Solubility | Soluble in DMSO | ||
| Chemical Name | [(2R,3R,4R,5R,6R)-4-[(2S,3R,4R,5R,6S)-4,5-dihydroxy-6-methyl-3-[(2S,3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyoxan-2-yl]oxy-6-[2-(3,4-dihydroxyphenyl)ethoxy]-5-hydroxy-2-(hydroxymethyl)oxan-3-yl] (E)-3-(3,4-dihydroxyphenyl)prop-2-enoate | ||
| SMILES | CC1C(C(C(C(O1)OC2C(C(OC(C2OC(=O)C=CC3=CC(=C(C=C3)O)O)CO)OCCC4=CC(=C(C=C4)O)O)O)OC5C(C(C(C(O5)CO)O)O)O)O)O | ||
| Standard InChIKey | SDRRSTAVRUERNC-GUTOYVNHSA-N | ||
| Standard InChI | InChI=1S/C35H46O20/c1-14-24(43)27(46)32(55-34-28(47)26(45)25(44)21(12-36)51-34)35(50-14)54-31-29(48)33(49-9-8-16-3-6-18(39)20(41)11-16)52-22(13-37)30(31)53-23(42)7-4-15-2-5-17(38)19(40)10-15/h2-7,10-11,14,21-22,24-41,43-48H,8-9,12-13H2,1H3/b7-4+/t14-,21+,22+,24-,25-,26-,27+,28+,29+,30+,31+,32+,33+,34-,35-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Teupolioside and verbascoside show anti-inflammatory and wound healing activities, they are extremely effective inhibitors of chemokine and growth factor expression by cultured human keratinocytes treated with pro-inflammatory cytokines, TNF-alpha and interferon-gamma. | |||||

Teupolioside Dilution Calculator

Teupolioside Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2711 mL | 6.3557 mL | 12.7113 mL | 25.4227 mL | 31.7783 mL |
| 5 mM | 0.2542 mL | 1.2711 mL | 2.5423 mL | 5.0845 mL | 6.3557 mL |
| 10 mM | 0.1271 mL | 0.6356 mL | 1.2711 mL | 2.5423 mL | 3.1778 mL |
| 50 mM | 0.0254 mL | 0.1271 mL | 0.2542 mL | 0.5085 mL | 0.6356 mL |
| 100 mM | 0.0127 mL | 0.0636 mL | 0.1271 mL | 0.2542 mL | 0.3178 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ethyl caproate
Catalog No.:BCN9832
CAS No.:123-66-0
- 28-Homobrassinolide
Catalog No.:BCN9831
CAS No.:82373-95-3
- Urushiol (15:1)
Catalog No.:BCN9830
CAS No.:35237-02-6
- 4,4'-Dimethoxychalcone
Catalog No.:BCN9829
CAS No.:2373-89-9
- Picrotoxinin
Catalog No.:BCN9828
CAS No.:17617-45-7
- 6-Hydroxyflavone
Catalog No.:BCN9827
CAS No.:6665-83-4
- Vaccarin E
Catalog No.:BCN9826
CAS No.:2252345-81-4
- Grayanotoxin I
Catalog No.:BCN9825
CAS No.:4720-09-6
- Acetic acid hexyl ester
Catalog No.:BCN9824
CAS No.:142-92-7
- Morindin
Catalog No.:BCN9823
CAS No.:60450-21-7
- Eclalbasaponin II
Catalog No.:BCN9822
CAS No.:78285-90-3
- Cimicifugic acid F
Catalog No.:BCN9821
CAS No.:220618-91-7
- BIX 01294 Trihydrochloride
Catalog No.:BCN9834
CAS No.:1392399-03-9
- 6,7-Bis(benzyloxy)coumarin
Catalog No.:BCN9835
CAS No.:909-84-2
- Urushiol (15:2)
Catalog No.:BCN9836
CAS No.:83258-37-1
- Isobutyl acetate
Catalog No.:BCN9837
CAS No.:110-19-0
- 5-Methoxyflavone
Catalog No.:BCN9838
CAS No.:42079-78-7
- Pilocarpine
Catalog No.:BCN9839
CAS No.:92-13-7
- Sutherlandioside D
Catalog No.:BCN9840
CAS No.:1055329-49-1
- Bletilol B
Catalog No.:BCN9841
CAS No.:147235-17-4
- Butyl acetate
Catalog No.:BCN9842
CAS No.:123-86-4
- Eugenol benzoate
Catalog No.:BCN9843
CAS No.:531-26-0
- Tryptanthrine
Catalog No.:BCN9844
CAS No.:13220-57-0
- 9-Hydroxy-O-senecioyl-8,9-dihydrooroselol
Catalog No.:BCN9845
CAS No.:31456-63-0
Direct inhibition of calcineurin by caffeoyl phenylethanoid glycosides from Teucrium chamaedrys and Nepeta cataria.[Pubmed:21843624]
J Ethnopharmacol. 2011 Oct 11;137(3):1306-10.
ETHNOPHARMACOLOGICAL RELEVANCE: Teucrium chamaedrys L. and Nepeta cataria L. (Lamiaceae) are species with traditional uses that relate to the treatment of inflammation. Extracts of both species were found to inhibit calcineurin; an important regulator of T-cell mediated inflammation that has received little attention in ethnopharmacological research. MATERIALS AND METHODS: Extracts and isolated compounds were tested against calcineurin in its calmodulin-activated and basal un-activated state. Active compounds were isolated using Sephadex LH-20 gel filtration and HPLC then identified using NMR spectroscopy. RESULTS AND CONCLUSIONS: Activity-guided fractionation of Teucrium chamaedrys and Nepeta cataria led to the isolation of the caffeoyl phenylethanoid glycosides teucrioside, verbascoside and lamiuside A (Teupolioside). The three compounds inhibited calcineurin both in the presence and absence of calmodulin, suggesting a direct interaction with calcineurin. Calcineurin inhibition should be considered as a potential mode of action when investigating the immunomodulatory activity of caffeoyl phenylethanoid glycoside containing plants.
Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression.[Pubmed:19723070]
Ann N Y Acad Sci. 2009 Aug;1171:305-13.
Oxidative stress is a common response of epidermal cells to a variety of noxious stimuli such as ultraviolet (UV) radiation from solar light and proinflammatory cytokines from skin-infiltrating leukocytes. Here, we report that two types of plant-derived antioxidants, the phenylpropanoid glycoside verbascoside as well as the flavonoids rutin and quercetin possess protective effects against UVC-induced cell damage and proinflammatory activation. The molecules under investigation were effective against the loss of cell integrity associated with necrosis in doses consistent with their antioxidant activity, whereas they did not significantly oppose UVC-induced proliferation arrest and apoptosis. By contrast, only verbascoside effectively inhibited cytokine-induced release of proinflammatory mediators in a dose-dependent fashion. Verbascoside and its homologue Teupolioside dramatically impaired NF-kappaB and AP-1 DNA binding activity. These results suggest that plant polyphenols with antioxidant properties have distinct mechanisms in the suppression of oxidative stress induced in keratinocytes by different stimuli. Verbascoside and Teupolioside are hence of potential interest in the protection of the skin from both environmental and inflammatory insults.
Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis.[Pubmed:19070602]
Biochem Pharmacol. 2009 Mar 1;77(5):845-57.
The aim of the present study was to examine the effects of phenylpropanoid glycoside, Teupolioside, biotechnologically produced by IRBN22 Ajuga reptans cell line, in rats subjected to experimental colitis. Colitis was induced in rats by intracolonic instillation of dinitrobenzene sulfonic acid (DNBS). Teupolioside was administered daily orally (0.2 or 2mgkg(-1)). On Day 4, animals were sacrificed and tissues were taken for histological and biochemical analysis. Four days after DNBS administration, colon TNF-alpha and IL-1beta productions were increased, associated with colon damage. Neutrophil infiltration, by myeloperoxidase activity, in the mucosa was associated with up-regulation of ICAM-1 and P-selectin and high levels of malondialdehyde. Immunohistochemistry for nitrotyrosine and poly (ADP-ribose) polymerase (PARP) showed an intense staining in the inflamed colon. Biochemical methods and zymography were used to analyze MMP-9 and -2 activities in colon tissues from DNBS-injured rats. Treatment with Teupolioside significantly reduced the appearance of diarrhoea and the loss of body weight. This was associated with a remarkable amelioration in the disruption of the colonic architecture and a significant reduction in colonic myeloperoxidase activity and malondialdehyde levels. Teupolioside also reduced the pro-inflammatory cytokines release, the appearance of nitrotyrosine and PARP immunoreactivity in the colon and reduced the up-regulation of ICAM-1 and the expression of P-selectin. Therefore, Teupolioside also reduced proMMP-9 and -2 activity induced in the colon by DNBS administration. The results of this study suggested that administration of Teupolioside may be beneficial for treatment of inflammatory bowel disease.
Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides.[Pubmed:17543237]
Cell Mol Biol (Noisy-le-grand). 2007 May 30;53(5):84-91.
Two phenylpropanoid glycosides, verbascoside (VB) and Teupolioside (TP), produced biotechnologically by Syringa vulgaris and Ajuga reptans plant cell cultures, were studied in vitro and in vivo for their anti-inflammatory and wound healing activities. It was shown that TP- and VB-containing extracts significantly accelerated wound healing and possessed remarkable anti-inflammatory action in the excision wound model. These effects correlated with the inhibition of reactive oxygen species release from the whole blood leukocytes and with the ferrous ion chelating capacity. On the other hand, they don't correlate either with free radical scavenging or with the inhibition of lipid peroxidation in the cell-free systems. Furthermore, both VB- and TP-containing extracts were extremely effective inhibitors of chemokine and growth factor expression by cultured human keratinocytes treated with pro-inflammatory cytokines, TNF-alpha and interferon-gamma.


