Grayanotoxin ICAS# 4720-09-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 4720-09-6 | SDF | Download SDF |
| PubChem ID | 9548612 | Appearance | White powder |
| Formula | C22H36O7 | M.Wt | 412.5 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Synonyms | Acetylandromedol; Andromedotoxin; Rhodotoxin | ||
| Solubility | Soluble in ethanol and methan | ||
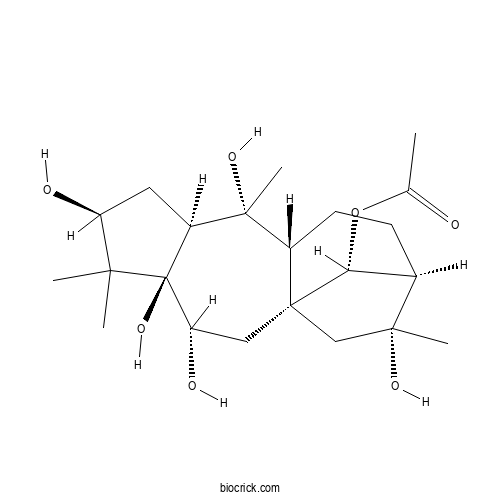
| Chemical Name | [(1S,3R,4R,6S,8S,9R,10R,13R,14R,16R)-3,4,6,9,14-pentahydroxy-5,5,9,14-tetramethyl-16-tetracyclo[11.2.1.01,10.04,8]hexadecanyl] acetate | ||
| SMILES | CC(=O)OC1C2CCC3C1(CC(C4(C(C3(C)O)CC(C4(C)C)O)O)O)CC2(C)O | ||
| Standard InChIKey | NXCYBYJXCJWMRY-VGBBEZPXSA-N | ||
| Standard InChI | InChI=1S/C22H36O7/c1-11(23)29-17-12-6-7-13-20(5,27)14-8-15(24)18(2,3)22(14,28)16(25)9-21(13,17)10-19(12,4)26/h12-17,24-28H,6-10H2,1-5H3/t12-,13+,14+,15+,16-,17-,19-,20-,21+,22+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Grayanotoxin I has an inhibitory effect on the initiation of action potentials of fibers of both muscles. This toxin causes a gradual decrease of the resting potential and an elevation of the critical firing level. | |||||

Grayanotoxin I Dilution Calculator

Grayanotoxin I Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4242 mL | 12.1212 mL | 24.2424 mL | 48.4848 mL | 60.6061 mL |
| 5 mM | 0.4848 mL | 2.4242 mL | 4.8485 mL | 9.697 mL | 12.1212 mL |
| 10 mM | 0.2424 mL | 1.2121 mL | 2.4242 mL | 4.8485 mL | 6.0606 mL |
| 50 mM | 0.0485 mL | 0.2424 mL | 0.4848 mL | 0.9697 mL | 1.2121 mL |
| 100 mM | 0.0242 mL | 0.1212 mL | 0.2424 mL | 0.4848 mL | 0.6061 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acetic acid hexyl ester
Catalog No.:BCN9824
CAS No.:142-92-7
- Morindin
Catalog No.:BCN9823
CAS No.:60450-21-7
- Eclalbasaponin II
Catalog No.:BCN9822
CAS No.:78285-90-3
- Cimicifugic acid F
Catalog No.:BCN9821
CAS No.:220618-91-7
- beta-Citronellol
Catalog No.:BCN9820
CAS No.:106-22-9
- trans-Fertaric acid
Catalog No.:BCN9819
CAS No.:74282-22-7
- Tricetin
Catalog No.:BCN9818
CAS No.:520-31-0
- Withanoside V
Catalog No.:BCN9817
CAS No.:256520-90-8
- 3-Aminocoumarin
Catalog No.:BCN9816
CAS No.:1635-31-0
- 7-Ethoxy-4-methylcoumarin
Catalog No.:BCN9815
CAS No.:87-05-8
- Vicinin 2
Catalog No.:BCN9814
CAS No.:90456-53-4
- 1,2,3-Tri-n-Octanoylglycerol
Catalog No.:BCN9813
CAS No.:538-23-8
- Vaccarin E
Catalog No.:BCN9826
CAS No.:2252345-81-4
- 6-Hydroxyflavone
Catalog No.:BCN9827
CAS No.:6665-83-4
- Picrotoxinin
Catalog No.:BCN9828
CAS No.:17617-45-7
- 4,4'-Dimethoxychalcone
Catalog No.:BCN9829
CAS No.:2373-89-9
- Urushiol (15:1)
Catalog No.:BCN9830
CAS No.:35237-02-6
- 28-Homobrassinolide
Catalog No.:BCN9831
CAS No.:82373-95-3
- Ethyl caproate
Catalog No.:BCN9832
CAS No.:123-66-0
- Teupolioside
Catalog No.:BCN9833
CAS No.:143617-02-1
- BIX 01294 Trihydrochloride
Catalog No.:BCN9834
CAS No.:1392399-03-9
- 6,7-Bis(benzyloxy)coumarin
Catalog No.:BCN9835
CAS No.:909-84-2
- Urushiol (15:2)
Catalog No.:BCN9836
CAS No.:83258-37-1
- Isobutyl acetate
Catalog No.:BCN9837
CAS No.:110-19-0
Rhodomicranosides A-I, analgesic diterpene glucosides with diverse carbon skeletons from Rhododendron micranthum.[Pubmed:30445297]
Phytochemistry. 2019 Feb;158:1-12.
Nine previously undescribed diterpene glucosides, rhodomicranosides A-I, comprising leucothane, 4,5-seco-ent-kaurane, and grayanane types, respectively, were isolated from the leaves of Rhododendron micranthum, along with seven known diterpenoids. Their structures were elucidated based on extensive spectroscopic analyses such as HRESIMS, 1D and 2D NMR, UV, and IR, and their absolute configurations were determined by various methods including X-ray diffraction analysis, electronic circular dichroism spectroscopy (ECD), calculated ECD, and Mo2(OAc)4-induced ECD, as well as chemical methods. This is the first time to report the crystal structures of leucothane diterpene glycosides. Rhodomicranosides A-C represent the first examples of 15alpha-hydroxy-leucothane diterpenoids, leucothane diterpene diglucosides, and 9beta-hydroxy-leucothane diterpenoids, respectively. Rhodomicranosides D and E are the second and third examples of 4,5-seco-ent-kaurane diterpenoids, and this is the first time to report 4,5-seco-ent-kaurane-type diterpenoids from the genus of Rhododendron. Rhodomicranosides F and G are the first examples of 5alpha-H-grayan-1(10),9(11)-diene-6-one diterpenoids. Some isolated diterpenoids were evaluated for their analgesic activity in an acetic acid-induced writhing test, and rhodomicranosides A-E and H, pierisformoside F, iso-Grayanotoxin II, and grayanotoxins I, III, and IV showed significant analgesic effects with the percentage inhibitions over 50% at the dose of 1.0mg/kg. In particular, grayanotoxins I and III exhibited more potent analgesic activity than morphine at a dose of 0.2mg/kg, and they showed significant analgesic activity even at a lower dose of 0.04mg/kg with the inhibition rates of 71.5% and 69.3%, respectively. Their preliminary structure-activity relationships were discussed.
Grayanotoxin I Intoxication in Pet Pigs.[Pubmed:30071802]
Vet Pathol. 2018 Nov;55(6):896-899.
Contaminated honey is a common cause of Grayanotoxin Intoxication in humans. Intoxication of animals, especially cattle, is usually due to ingestion of plants of the Ericaceae family, such as Rhododendron. Here, we report the ingestion of Pieris japonica as the cause of Grayanotoxin I intoxication in 2 miniature pigs that were kept as pets. The pigs showed sudden onset of pale oral mucosa, tachycardia, tachypnea, hypersalivation, tremor, and ataxia that progressed to lateral recumbency. The pathological examination of one pig revealed no specific indications for intoxication except for the finding of plant material of Pieris japonica in the intestine. Grayanotoxin I was identified in the ingested plant, gastric content, blood, liver, bile, kidney, urine, lung, and skeletal muscle via HPLC-MS/MS. Grayanotoxin I should be considered as a differential etiological diagnosis in pigs with unspecific signs and discovery of ingested plant material as the only indication in the pathologic examination.
Determination of Grayanotoxins from Rhododendron brachycarpum in Dietary Supplements and Homemade Wine by Liquid Chromatography-Quadrupole Time-of-Flight-Mass Spectrometry and Liquid Chromatography-Tandem Mass Spectrometry.[Pubmed:29433311]
J Agric Food Chem. 2018 Feb 28;66(8):1935-1940.
A sensitive and specific high-performance liquid chromatography-quadrupole time-of-flight-mass spectrometry (LC-QTOF-MS) method combined with liquid chromatography-tandem mass spectrometry (LC-MS/MS) was developed for the determination of grayanotoxins I and III in dietary supplements and homemade wine. Grayanotoxins I and III were successfully extracted using solid-phase extraction cartridges, characterized by LC-QTOF-MS, and quantitated by LC-MS/MS. The LC-MS/MS calibration curves were linear over concentrations of 10-100 ng/mL (Grayanotoxin I) and 20-400 ng/mL (Grayanotoxin III). Grayanotoxins I and III were found in 51 foodstuffs, with quantitative determinations revealing total toxin concentrations of 18.4-101000 ng/mL (Grayanotoxin I) and 15.3-56000 ng/mL (Grayanotoxin III). The potential of the validated method was demonstrated by successful quantitative analysis of grayanotoxins I and III in dietary supplements and homemade wine; the method appears suitable for the routine detection of grayanotoxins I and III from Rhododendron brachycarpum.
Leaf trichomes and foliar chemistry mediate defence against glasshouse thrips; Heliothrips haemorrhoidalis (Bouche) in Rhododendron simsii.[Pubmed:32480536]
Funct Plant Biol. 2016 Dec;43(12):1170-1182.
Herbivore defence mechanisms are a costly diversion of resources away from growth and reproduction. Thus time-limited and tissue specific expression in critical plant parts is more efficient as defined by optimal defence theory. Surprisingly little is known about Rhododendron herbivore defence but it may be mediated by combined chemical and physical mechanisms. Rhododendron simsii Planch. survives cyclic infestations of a leaf-feeding thrips, Heliothrips haemorrhoidalis (Bouche), which severely damage mature leaves but avoid terminal young leaves suggesting specific, localised defence expression. We examined correlations between the distribution of thrips and feeding damage with density of trichomes and the concentration of the diterpenoid, Grayanotoxin I, a compound implicated in but not previously reported to mediate invertebrate defence in Rhododendron. Our data show that as leaves matured the number of thrips and area of feeding damage increased as trichome density and Grayanotoxin I concentration decreased, this inverse correlation suggesting trichomes and Grayanotoxin I mediate defence in younger leaf tissue. Grayanotoxin I was tested against H. haemorrhoidalis and was toxic to immature life stages and repellent to the adult thrips, reducing numbers of first instars emerging on leaves when applied at ecologically relevant concentrations. This work demonstrates that the pattern of defensive traits in foliage of a species of Rhododendron is key to its ability to tolerate cyclic infestations of a generalist herbivore, effectively conserving vital tissues required for growth and reproduction.
Acute effects of grayanotoxin in rhododendron honey on kidney functions in rats.[Pubmed:26490905]
Environ Sci Pollut Res Int. 2016 Feb;23(4):3300-9.
The aim of the study is to evaluate the acute biochemical and histological changes in rat kidneys after treatment with grayanotoxin (GTX) of rhododendron honey (RH). A total of 60 Sprague-Dawley female rats were divided into five groups of 12 rats each, one being a control group (group 1) and group 2 was treated with 0.015 mg/kg/bw of GTX standard preparation via intraperitoneal injection. Groups 3, 4, and 5 were given RH at doses of 0.1, 0.5, and 2.5 g/kg/bw, respectively, via oral gavage. Compared to the control group, significant increases were observed in glucose, blood urea nitrogen (BUN), and creatinine levels of the GTX-injected groups after 1 h. However, in low dose RH group, such an increase was not observed and had a normal appearance histologically. Therefore, low dose (1 g/kg/bw) of RH produces no acute adverse effects on renal functions of rats.
Pyrethroids and Nectar Toxins Have Subtle Effects on the Motor Function, Grooming and Wing Fanning Behaviour of Honeybees (Apis mellifera).[Pubmed:26280999]
PLoS One. 2015 Aug 17;10(8):e0133733.
Sodium channels, found ubiquitously in animal muscle cells and neurons, are one of the main target sites of many naturally-occurring, insecticidal plant compounds and agricultural pesticides. Pyrethroids, derived from compounds found only in the Asteraceae, are particularly toxic to insects and have been successfully used as pesticides including on flowering crops that are visited by pollinators. Pyrethrins, from which they were derived, occur naturally in the nectar of some flowering plant species. We know relatively little about how such compounds--i.e., compounds that target sodium channels--influence pollinators at low or sub-lethal doses. Here, we exposed individual adult forager honeybees to several compounds that bind to sodium channels to identify whether these compounds affect motor function. Using an assay previously developed to identify the effect of drugs and toxins on individual bees, we investigated how acute exposure to 10 ng doses (1 ppm) of the pyrethroid insecticides (cyfluthrin, tau-fluvalinate, allethrin and permethrin) and the nectar toxins (aconitine and Grayanotoxin I) affected honeybee locomotion, grooming and wing fanning behaviour. Bees exposed to these compounds spent more time upside down and fanning their wings. They also had longer bouts of standing still. Bees exposed to the nectar toxin, aconitine, and the pyrethroid, allethrin, also spent less time grooming their antennae. We also found that the concentration of the nectar toxin, Grayanotoxin I (GTX), fed to bees affected the time spent upside down (i.e., failure to perform the righting reflex). Our data show that low doses of pyrethroids and other nectar toxins that target sodium channels mainly influence motor function through their effect on the righting reflex of adult worker honeybees.
Cardiac Effects of Mad Honey Poisoning and Its Management in Emergency Department: A Review from Turkey.[Pubmed:25613735]
Cardiovasc Toxicol. 2016 Jan;16(1):1-4.
Mad honey poisoning occurs when honey containing Grayanotoxin Is digested. The most common clinical signs and symptoms of poisoning involve findings of digestive system irritation, severe bradycardia and hypotension and central nervous system reaction. In this review, we aimed to underline the cardiac effects of mad honey poisoning. We also aimed to raise the awareness of physicians about early diagnosis and treatment of this rare entity.
Extracts from Rhododendron ferrugineum do not exhibit grayanotoxin I: an analytical survey on grayanotoxin I within the genus Rhododendron.[Pubmed:25215540]
Planta Med. 2014 Oct;80(15):1321-8.
For quantitative determination of Grayanotoxin I (1) in plant material, a GC/MS method was developed after trimethylsilyl derivatisation of the analytes. Forskolin (5) was used as an internal standard for quantification. ICH-compliant method validation indicated sufficient specificity, precision, quantitation (15 microg/mL) and detection (5 microg/mL) limits. Regression analysis showed that a non-linear (polynomial) model was preferable to a linear one. For isolation of Grayanotoxin I reference material from Rhododendron ponticum leaves, an efficient two-step fast centrifugal partition chromatography isolation protocol is described. A survey of 17 different plant species from the genus Rhododendron revealed high Grayanotoxin I content for R. catawbiense, R. ponticum, R. degronianum subsp. yakushimanum, R. x sochadzeae, R. moupinense, R. galactinum, and R. mucronatum var. ripense. The content of this compound in leaf material from R. ponticum decreased rapidly during drying process. Grayanotoxin I was not detected in different batches of fresh leaves and fruits from R. ferrugineum. In contrast to the claims of German health authorities, this traditionally used herb therefore cannot be evaluated as toxic due to the presence of Grayanotoxin I.


