MorindinCAS# 60450-21-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 60450-21-7 | SDF | Download SDF |
| PubChem ID | 151621 | Appearance | Yellow powder |
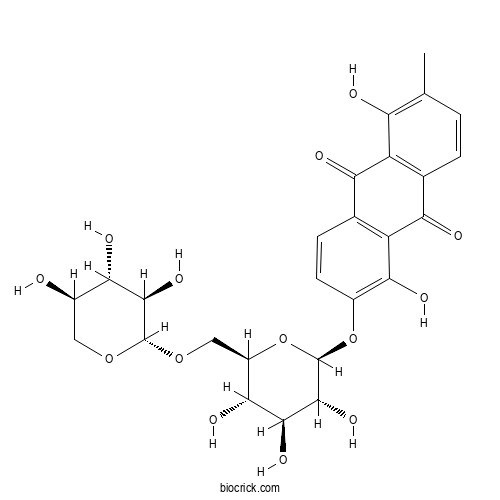
| Formula | C26H28O14 | M.Wt | 564.5 |
| Type of Compound | Anthraquinones | Storage | Desiccate at -20°C |
| Synonyms | Morindone 3-O-β-primeveroside | ||
| Solubility | Soluble in methanol; slightly soluble in water | ||
| Chemical Name | 1,5-dihydroxy-2-methyl-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxymethyl]oxan-2-yl]oxyanthracene-9,10-dione | ||
| SMILES | CC1=C(C2=C(C=C1)C(=O)C3=C(C2=O)C=CC(=C3O)OC4C(C(C(C(O4)COC5C(C(C(CO5)O)O)O)O)O)O)O | ||
| Standard InChIKey | UVLAQGRQOILFBG-UHCLWRNRSA-N | ||
| Standard InChI | InChI=1S/C26H28O14/c1-8-2-3-9-14(16(8)28)17(29)10-4-5-12(20(32)15(10)18(9)30)39-26-24(36)22(34)21(33)13(40-26)7-38-25-23(35)19(31)11(27)6-37-25/h2-5,11,13,19,21-28,31-36H,6-7H2,1H3/t11-,13-,19+,21-,22+,23-,24-,25+,26-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

Morindin Dilution Calculator

Morindin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7715 mL | 8.8574 mL | 17.7148 mL | 35.4296 mL | 44.287 mL |
| 5 mM | 0.3543 mL | 1.7715 mL | 3.543 mL | 7.0859 mL | 8.8574 mL |
| 10 mM | 0.1771 mL | 0.8857 mL | 1.7715 mL | 3.543 mL | 4.4287 mL |
| 50 mM | 0.0354 mL | 0.1771 mL | 0.3543 mL | 0.7086 mL | 0.8857 mL |
| 100 mM | 0.0177 mL | 0.0886 mL | 0.1771 mL | 0.3543 mL | 0.4429 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Eclalbasaponin II
Catalog No.:BCN9822
CAS No.:78285-90-3
- Cimicifugic acid F
Catalog No.:BCN9821
CAS No.:220618-91-7
- beta-Citronellol
Catalog No.:BCN9820
CAS No.:106-22-9
- trans-Fertaric acid
Catalog No.:BCN9819
CAS No.:74282-22-7
- Tricetin
Catalog No.:BCN9818
CAS No.:520-31-0
- Withanoside V
Catalog No.:BCN9817
CAS No.:256520-90-8
- 3-Aminocoumarin
Catalog No.:BCN9816
CAS No.:1635-31-0
- 7-Ethoxy-4-methylcoumarin
Catalog No.:BCN9815
CAS No.:87-05-8
- Vicinin 2
Catalog No.:BCN9814
CAS No.:90456-53-4
- 1,2,3-Tri-n-Octanoylglycerol
Catalog No.:BCN9813
CAS No.:538-23-8
- Norcamphor
Catalog No.:BCN9812
CAS No.:497-38-1
- 3-Hydroxycoumarin
Catalog No.:BCN9811
CAS No.:939-19-5
- Acetic acid hexyl ester
Catalog No.:BCN9824
CAS No.:142-92-7
- Grayanotoxin I
Catalog No.:BCN9825
CAS No.:4720-09-6
- Vaccarin E
Catalog No.:BCN9826
CAS No.:2252345-81-4
- 6-Hydroxyflavone
Catalog No.:BCN9827
CAS No.:6665-83-4
- Picrotoxinin
Catalog No.:BCN9828
CAS No.:17617-45-7
- 4,4'-Dimethoxychalcone
Catalog No.:BCN9829
CAS No.:2373-89-9
- Urushiol (15:1)
Catalog No.:BCN9830
CAS No.:35237-02-6
- 28-Homobrassinolide
Catalog No.:BCN9831
CAS No.:82373-95-3
- Ethyl caproate
Catalog No.:BCN9832
CAS No.:123-66-0
- Teupolioside
Catalog No.:BCN9833
CAS No.:143617-02-1
- BIX 01294 Trihydrochloride
Catalog No.:BCN9834
CAS No.:1392399-03-9
- 6,7-Bis(benzyloxy)coumarin
Catalog No.:BCN9835
CAS No.:909-84-2
Colouring of Pacific barkcloths: identification of the brown, red and yellow colourants used in the decoration of historic Pacific barkcloths.[Pubmed:31258912]
Herit Sci. 2019;7(1):2.
Barkcloth textiles made in the Pacific islands and collected by western explorers in the eighteenth and nineteenth centuries form part of many museum collections worldwide. Here high-performance liquid chromatography (HPLC) and X-ray fluorescence (XRF) were used on cloths that were highly coloured or pigmented specifically focussing on identifying the red, yellow and brown colorants. The cloths studied came from collections held at the Hunterian, University of Glasgow, the Economic Botany Collection, Royal Botanic Gardens Kew and the Centre for Textile Conservation and Technical Art History, University of Glasgow. HPLC analysis was carried out following a sequential extraction procedure to minimise changes to the colorants during extraction. A portable XRF was used so no invasive sampling was required. A small number of plant derived colorants were found, Morinda citrifolia, noni (Morindin or morindone), Rubia tinctorum (madder), tree tannins and Curcuma longa (turmeric) plus an inorganic colorant, iron oxide. For 40 samples a single colorant was found while in the remaining 12 samples combinations of up to three colorants were found. Madder was found in only 2 samples on the same cloth. The morindone coloured samples were all red whereas Morindin samples were both red and yellow. Morindin was used predominantly in combination with other colouring agents. A combination of iron ochre and organic colorant was found in 4 samples. These findings show that despite the numerous potential colorant sources for red, brown and yellow shades listed in the many accounts of historic barkcloth making, only five types of plant colourant and one inorganic pigment were found. There are a number of potential reasons for these findings. Some colours may have faded and so no longer appear coloured. It is also possible that, as some of these cloths were prepared specifically as gifts for visitors or for ceremonial uses, the makers used materials that they knew would retain their integrity over time. Perhaps, like artisans worldwide, experience had taught them that some colorants, although initially bright and vivid, faded over time.


