4,4'-DimethoxychalconeCAS# 2373-89-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2373-89-9 | SDF | Download SDF |
| PubChem ID | 5377817 | Appearance | Powder |
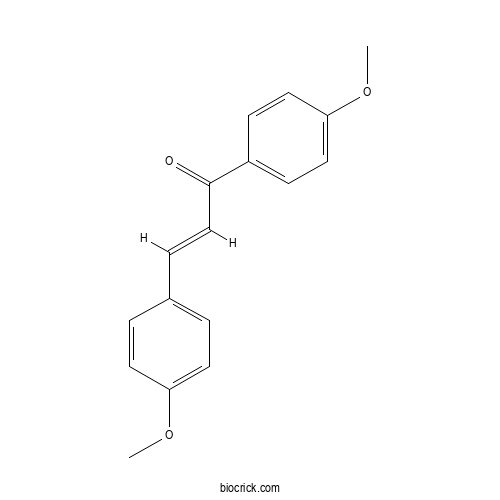
| Formula | C17H16O3 | M.Wt | 268.3 |
| Type of Compound | Chalcones | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-1,3-bis(4-methoxyphenyl)prop-2-en-1-one | ||
| SMILES | COC1=CC=C(C=C1)C=CC(=O)C2=CC=C(C=C2)OC | ||
| Standard InChIKey | HDXVSZWKIHQDES-LFYBBSHMSA-N | ||
| Standard InChI | InChI=1S/C17H16O3/c1-19-15-8-3-13(4-9-15)5-12-17(18)14-6-10-16(20-2)11-7-14/h3-12H,1-2H3/b12-5+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Reference standards. | |||||

4,4'-Dimethoxychalcone Dilution Calculator

4,4'-Dimethoxychalcone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7272 mL | 18.6359 mL | 37.2717 mL | 74.5434 mL | 93.1793 mL |
| 5 mM | 0.7454 mL | 3.7272 mL | 7.4543 mL | 14.9087 mL | 18.6359 mL |
| 10 mM | 0.3727 mL | 1.8636 mL | 3.7272 mL | 7.4543 mL | 9.3179 mL |
| 50 mM | 0.0745 mL | 0.3727 mL | 0.7454 mL | 1.4909 mL | 1.8636 mL |
| 100 mM | 0.0373 mL | 0.1864 mL | 0.3727 mL | 0.7454 mL | 0.9318 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Picrotoxinin
Catalog No.:BCN9828
CAS No.:17617-45-7
- 6-Hydroxyflavone
Catalog No.:BCN9827
CAS No.:6665-83-4
- Vaccarin E
Catalog No.:BCN9826
CAS No.:2252345-81-4
- Grayanotoxin I
Catalog No.:BCN9825
CAS No.:4720-09-6
- Acetic acid hexyl ester
Catalog No.:BCN9824
CAS No.:142-92-7
- Morindin
Catalog No.:BCN9823
CAS No.:60450-21-7
- Eclalbasaponin II
Catalog No.:BCN9822
CAS No.:78285-90-3
- Cimicifugic acid F
Catalog No.:BCN9821
CAS No.:220618-91-7
- beta-Citronellol
Catalog No.:BCN9820
CAS No.:106-22-9
- trans-Fertaric acid
Catalog No.:BCN9819
CAS No.:74282-22-7
- Tricetin
Catalog No.:BCN9818
CAS No.:520-31-0
- Withanoside V
Catalog No.:BCN9817
CAS No.:256520-90-8
- Urushiol (15:1)
Catalog No.:BCN9830
CAS No.:35237-02-6
- 28-Homobrassinolide
Catalog No.:BCN9831
CAS No.:82373-95-3
- Ethyl caproate
Catalog No.:BCN9832
CAS No.:123-66-0
- Teupolioside
Catalog No.:BCN9833
CAS No.:143617-02-1
- BIX 01294 Trihydrochloride
Catalog No.:BCN9834
CAS No.:1392399-03-9
- 6,7-Bis(benzyloxy)coumarin
Catalog No.:BCN9835
CAS No.:909-84-2
- Urushiol (15:2)
Catalog No.:BCN9836
CAS No.:83258-37-1
- Isobutyl acetate
Catalog No.:BCN9837
CAS No.:110-19-0
- 5-Methoxyflavone
Catalog No.:BCN9838
CAS No.:42079-78-7
- Pilocarpine
Catalog No.:BCN9839
CAS No.:92-13-7
- Sutherlandioside D
Catalog No.:BCN9840
CAS No.:1055329-49-1
- Bletilol B
Catalog No.:BCN9841
CAS No.:147235-17-4
Natural and Semisynthetic Chalcones as Dual FLT3 and Microtubule Polymerization Inhibitors.[Pubmed:32975953]
J Nat Prod. 2020 Oct 23;83(10):3111-3121.
Activating mutations in FLT3 receptor tyrosine kinase are found in a third of acute myeloid leukemia (AML) patients and are associated with disease relapse and a poor prognosis. The majority of these mutations are internal tandem duplications (ITDs) in the juxtamembrane domain of FLT3, which have been validated as a therapeutic target. The clinical success of selective inhibitors targeting oncogenic FLT3, however, has been limited due to the acquisition of drug resistance. Herein the identification of a dual FLT3/microtubule polymerization inhibitor, chalcone 4 (2'-allyloxy-4,4'-dimethoxychalcone), is reported through screening of 15 related chalcones for differential antiproliferative activity in leukemia cell lines dependent on FLT3-ITD (MV-4-11) or BCR-ABL (K562) oncogenes and by subsequent screening for mitotic inducers in the HCT116 cell line. Three natural chalcones (1-3) were found to be differentially more potent toward the MV-4-11 (FLT3-ITD) cell line compared to the K562 (BCR-ABL) cell line. Notably, the new semisynthetic chalcone 4, which is a 2'-O-allyl analogue of the natural chalcone 3, was found to be more potent toward the FLT3-ITD+ cell line and inhibited FLT3 signaling in FLT3-dependent cells. An in vitro kinase assay confirmed that chalcone 4 directly inhibited FLT3. Moreover, chalcone 4 induced mitotic arrest in these cells and inhibited tubulin polymerization in both cellular and biochemical assays. Treatment of MV-4-11 cells with this inhibitor for 24 and 48 h resulted in apoptotic cell death. Finally, chalcone 4 was able to overcome TKD mutation-mediated acquired resistance to FLT3 inhibitors in a MOLM-13 cell line expressing FLT3-ITD with the D835Y mutation. Chalcone 4 is, therefore, a promising lead for the discovery of dual-target FLT3 inhibitors.
4,4'Dimethoxychalcone: a natural flavonoid that promotes health through autophagy-dependent and -independent effects.[Pubmed:31248332]
Autophagy. 2019 Sep;15(9):1662-1664.
The age-induced deterioration of the organism results in detrimental and ultimately lethal pathologies. The process of aging itself involves a plethora of different mechanisms that should be subverted concurrently to delay and/or prevent age-related maladies. We have identified a natural compound, 4,4'-dimethoxychalcone (DMC), which promotes longevity in yeast, worms and flies, and protects mice from heart injury and liver toxicity. Interestingly, both the DMC-mediated lifespan extension and the cardioprotection depend on macroautophagy/autophagy whereas hepatoprotection does not. DMC induces autophagy by inhibiting specific GATA transcription factors (TFs), independently of the TORC1 kinase pathway. The autophagy-independent beneficial effects of DMC might involve its antioxidative properties. DMC treatment results in a phylogenetically conserved, systemic impact on the metabolome, which is most prominently characterized by changes in cellular amino acid composition. Altogether, DMC exerts multiple, geroprotective effects by igniting distinct pathways, and thus represents a potential pharmacological agent that delays aging through multipronged effects.
The flavonoid 4,4'-dimethoxychalcone promotes autophagy-dependent longevity across species.[Pubmed:30783116]
Nat Commun. 2019 Feb 19;10(1):651.
Ageing constitutes the most important risk factor for all major chronic ailments, including malignant, cardiovascular and neurodegenerative diseases. However, behavioural and pharmacological interventions with feasible potential to promote health upon ageing remain rare. Here we report the identification of the flavonoid 4,4'-dimethoxychalcone (DMC) as a natural compound with anti-ageing properties. External DMC administration extends the lifespan of yeast, worms and flies, decelerates senescence of human cell cultures, and protects mice from prolonged myocardial ischaemia. Concomitantly, DMC induces autophagy, which is essential for its cytoprotective effects from yeast to mice. This pro-autophagic response induces a conserved systemic change in metabolism, operates independently of TORC1 signalling and depends on specific GATA transcription factors. Notably, we identify DMC in the plant Angelica keiskei koidzumi, to which longevity- and health-promoting effects are ascribed in Asian traditional medicine. In summary, we have identified and mechanistically characterised the conserved longevity-promoting effects of a natural anti-ageing drug.
[Chemical constituents from leaves of Nelumbo nucifera].[Pubmed:23724680]
Zhongguo Zhong Yao Za Zhi. 2013 Mar;38(5):703-8.
To study the chemical constituents, twenty-seven compounds were isolated from the 70% ethanol extract from leaves of Nelumbo nucifera by modern chromatographic techniques. Their structures were identified as 10-octacosanol (1), beta-sitosterol (2), 1-undecanol (3), 1-eicosanol (4), daucosterol (5), 6'-hydroxy-4,4'-dimethoxychalcone (6), 3,7,8-trimethoxy-1-hydroxy-xanthone (7), rhamnetin-3-O-beta-D-glucopyranoside (8), chrysoeriol-7-O-beta-D-glucoside (9), quercetin-3-O-beta-D-glucopyranoside (10), quercetin-3-O-alpha-L-rhamnopyranosyl (11), hyperoside (12), quercetin-3-O-rutinoside (13), astragalin (14), isorhamnetin-3-O-alpha-L-rhamnopyranosyl-(1--> 6)-[alpha-D-lyxopyranosyl-(1 --> 2) -beta-D-glucopyranoside] (15), isorhamnetin-3-O-alpha-D-lyxopyranosyl-(1 --> 2) -beta-D-glucopyranoside (16), isorhamnetin-3-O-beta-D-glucopyranoside (17), isorhamnetin-3-O-alpha-L-rhamnopyranosyl-(1 --> 6)-beta-D-glucopyranoside (18), quercetin (19), kaempferol (20), dehydronuciferine (21), roemerine (22), stigmast-7-en-3-O-beta-D-glucopyranoside (23), stigmast-7-en-3beta-ol (24), and benzene-1,2-diol (25) on the basis of spectral data analysis. Compounds 1, 6, 7, 8, 24 and 25 were isolated from this plant for the first time, and compounds 15-18 were isolated from the leaves for the first time. Compounds 6, 8, 10, 11, 13 and 15 showed inhibitory activities against beta amyloid (1-42) by A-beta aggregation method with inhibition rates of (63.99 +/- 24.29)%, (79.61 +/- 4.49)%, (49.96 +/- 12.61)%, (101.19 +/- 8.19)%, (88.41+/-6.76)% and (72.48 +/- 8.97)%, respectively.
The regulation of inflammatory cytokine secretion in macrophage cell line by the chemical constituents of Rhus sylvestris.[Pubmed:19447618]
Bioorg Med Chem Lett. 2009 Jul 1;19(13):3607-10.
In our preliminary screening study on the anti-inflammatory activity, eight triterpenes, one sterol, and one chalcone were isolated from the CH(2)Cl(2)-soluble extract of the stems and leaves of Rhus sylvestris Siebold and Zucc (Anacardiaceae). On the basis of their spectroscopic data, these compounds were identified as 10alpha-cucurbitadienol (1), glut-5-en-3-ol (2), beta-amyrin acetate (3), beta-amyrin (4) and lupeol (5), cycloart-24-en-3-one (6), cycloart-25-en-3,24-dione (7), 24-hydroxycycloart-25-en-3-one (8), beta-sitosterol (9), and 2'-hydroxy-4,4'-dimethoxychalcone (10). All of them were isolated from this plant for the first. Furthermore, the compounds in non-cytotoxic concentrations (0-1.0microM) were tested for their ability to block inflammatory cytokine secretion in the presence of LPS in the murine RAW264.7 macrophage cell line. Among the compounds that were tested, compounds 8 and 9 reduced the LPS-induced secretion of IL-6, as well as TNF-alpha, in a mouse RAW264.7 macrophage cell line. Moreover, compounds 2, 3, 7, and 10 specifically diminished only the secretion of TNF-alpha even in 0.01microM concentrations. It is thus suggested that they are potential therapeutics of TNF-alpha-related diseases and conditions, such as transplant rejection, type II diabetes, and atherosclerosis.
Allergy-preventive flavonoids from Xanthorrhoea hastilis.[Pubmed:17409571]
Chem Pharm Bull (Tokyo). 2007 Apr;55(4):675-8.
Allergy-preventive activity was demonstrated for an extract of resins from Xanthorrhoea hastilis R. BR. in a search for allergy-preventive substances from natural sources. By bioassay-directed fractionation of this plant extract, a new flavanone, 3',5'-dihydroxy-7,4'-dimethoxyflavanone (1), and two new chalcones, 3,5,2'-trihydroxy-4,4'-dimethoxychalcone (2) and 5,2'-dihydroxy-3,4,4'-trimethoxychalcone (3), were isolated together with five known compounds, 5'-hydroxy-7,3',4'-trimethoxyflavanone (4), 3'-hydroxy-7,4'-dimethoxyflavanone (5), liquiritigenin 7-methyl ether (6), 4,2'-dihydroxy-4'-methoxychalcone (7) and sakuranetin (8). The structures of 1, 2 and 3 were elucidated by spectroscopic methods. All of these compounds showed allergy-preventive effects.


