Cyclo(L-Phe-L-Pro)CAS# 3705-26-8 |
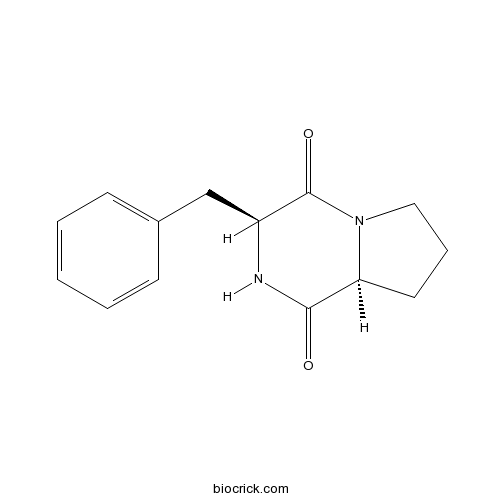
- Cyclo(Phe-Pro)
Catalog No.:BCN2416
CAS No.:14705-60-3
- Cyclo(D-Phe-L-Pro)
Catalog No.:BCN4011
CAS No.:26488-24-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3705-26-8 | SDF | Download SDF |
| PubChem ID | 443440 | Appearance | Oil |
| Formula | C14H16N2O2 | M.Wt | 244.3 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,8aS)-3-benzyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| SMILES | C1CC2C(=O)NC(C(=O)N2C1)CC3=CC=CC=C3 | ||
| Standard InChIKey | QZBUWPVZSXDWSB-RYUDHWBXSA-N | ||
| Standard InChI | InChI=1S/C14H16N2O2/c17-13-12-7-4-8-16(12)14(18)11(15-13)9-10-5-2-1-3-6-10/h1-3,5-6,11-12H,4,7-9H2,(H,15,17)/t11-,12-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Cyclo(L-Phe-L-Pro) shows antifungal and broad spectrum antibacterial activities. 2. Cyclo-(L-Phe-L-Pro) exhibits activity against methicillin-resistant S. aureus ATCC 43300 and Enterococcus raffinosus, with low toxicity against human hepatoma HepaRG cells. |
| Targets | Antifection |

Cyclo(L-Phe-L-Pro) Dilution Calculator

Cyclo(L-Phe-L-Pro) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0933 mL | 20.4666 mL | 40.9333 mL | 81.8666 mL | 102.3332 mL |
| 5 mM | 0.8187 mL | 4.0933 mL | 8.1867 mL | 16.3733 mL | 20.4666 mL |
| 10 mM | 0.4093 mL | 2.0467 mL | 4.0933 mL | 8.1867 mL | 10.2333 mL |
| 50 mM | 0.0819 mL | 0.4093 mL | 0.8187 mL | 1.6373 mL | 2.0467 mL |
| 100 mM | 0.0409 mL | 0.2047 mL | 0.4093 mL | 0.8187 mL | 1.0233 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Carbetocin
Catalog No.:BCC6304
CAS No.:37025-55-1
- (-)-Variabilin
Catalog No.:BCN4815
CAS No.:370102-93-5
- Zinterol hydrochloride
Catalog No.:BCC6911
CAS No.:37000-20-7
- FCCP
Catalog No.:BCC5659
CAS No.:370-86-5
- TMPyP4 tosylate
Catalog No.:BCC7899
CAS No.:36951-72-1
- Oroxylin A 7-O-beta-D-glucuronide
Catalog No.:BCN2337
CAS No.:36948-76-2
- Icilin
Catalog No.:BCC4074
CAS No.:36945-98-9
- Hydramicromelin B
Catalog No.:BCN7560
CAS No.:369391-55-9
- 6-epi-Augustifolin
Catalog No.:BCN3233
CAS No.:369390-94-3
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- p-Coumaryl alcohol
Catalog No.:BCN3922
CAS No.:3690-05-9
- TC 14012
Catalog No.:BCC7910
CAS No.:368874-34-4
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
- Z-Glu-OBzl
Catalog No.:BCC2780
CAS No.:3705-42-8
- Procyanidin C1
Catalog No.:BCN6317
CAS No.:37064-30-5
- Azlocillin sodium salt
Catalog No.:BCC4763
CAS No.:37091-65-9
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- Murrangatin
Catalog No.:BCN5426
CAS No.:37126-91-3
- 4'-Amino-3',5'-dichloroacetophenone
Catalog No.:BCC8678
CAS No.:37148-48-4
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- YM201636
Catalog No.:BCC4996
CAS No.:371942-69-7
- ZAPA sulfate
Catalog No.:BCC6563
CAS No.:371962-01-5
Isolation, Purification, and Characterization of Five Active Diketopiperazine Derivatives from Endophytic Streptomyces SUK 25 with Antimicrobial and Cytotoxic Activities.[Pubmed:28535606]
J Microbiol Biotechnol. 2017 Jul 28;27(7):1249-1256.
In our search for new sources of bioactive secondary metabolites from Streptomyces sp., the ethyl acetate extracts from endophytic Streptomyces SUK 25 afforded five active diketopiperazine (DKP) compounds. The aim of this study was to characterize the bioactive compounds isolated from endophytic Streptomyces SUK 25 and evaluate their bioactivity against multiple drug resistance (MDR) bacteria such as Enterococcus raffinosus, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter spp., and their cytotoxic activities against the human hepatoma (HepaRG) cell line. The production of secondary metabolites by this strain was optimized through Thornton's medium. Isolation, purification, and identification of the bioactive compounds were carried out using high-performance liquid chromatography, high-resolution mass liquid chromatography-mass spectrometry, Fourier transform infrared spectroscopy, and nuclear magnetic resonance, and cryopreserved HepaRG cells were selected to test the cytotoxicity. The results showed that endophytic Streptomyces SUK 25 produces four active DKP compounds and an acetamide derivative, which were elucidated as cyclo-(L-Val-L-Pro), cyclo-(L-Leu-L-Pro), cyclo-(L-Phe-L-Pro), cyclo-(L-Val-L-Phe), and N-(7-hydroxy-6-methyl-octyl)-acetamide. These active compounds exhibited activity against methicillin-resistant S. aureus ATCC 43300 and Enterococcus raffinosus, with low toxicity against human hepatoma HepaRG cells. Endophytic Streptomyces SUK 25 has the ability to produce DKP derivatives biologically active against some MDR bacteria with relatively low toxicity against HepaRG cells line.
Lactobacillus plantarum MiLAB 393 produces the antifungal cyclic dipeptides cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid.[Pubmed:12200282]
Appl Environ Microbiol. 2002 Sep;68(9):4322-7.
We have isolated a Lactobacillus plantarum strain (MiLAB 393) from grass silage that produces broad-spectrum antifungal compounds, active against food- and feed-borne filamentous fungi and yeasts in a dual-culture agar plate assay. Fusarium sporotrichioides and Aspergillus fumigatus were the most sensitive among the molds, and Kluyveromyces marxianus was the most sensitive yeast species. No inhibitory activity could be detected against the mold Penicillium roqueforti or the yeast Zygosaccharomyces bailii. An isolation procedure, employing a microtiter well spore germination bioassay, was devised to isolate active compounds from culture filtrate. Cell-free supernatant was fractionated on a C(18) SPE column, and the 95% aqueous acetonitrile fraction was further separated on a preparative HPLC C(18) column. Fractions active in the bioassay were then fractionated on a porous graphitic carbon column. The structures of the antifungal compounds Cyclo(L-Phe-L-Pro), cyclo(L-Phe-trans-4-OH-L-Pro) and 3-phenyllactic acid (L/D isomer ratio, 9:1), were determined by nuclear magnetic resonance spectroscopy, mass spectrometry, and gas chromatography. MIC values against A. fumigatus and P. roqueforti were 20 mg ml(-1) for Cyclo(L-Phe-L-Pro) and 7.5 mg ml(-1) for phenyllactic acid. Combinations of the antifungal compounds revealed weak synergistic effects. The production of the antifungal cyclic dipeptides Cyclo(L-Phe-L-Pro) and cyclo(L-Phe-trans-4-OH-L-Pro) by lactic acid bacteria is reported here for the first time.


