p-Coumaryl alcoholCAS# 3690-05-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 3690-05-9 | SDF | Download SDF |
| PubChem ID | 5280535 | Appearance | White powder |
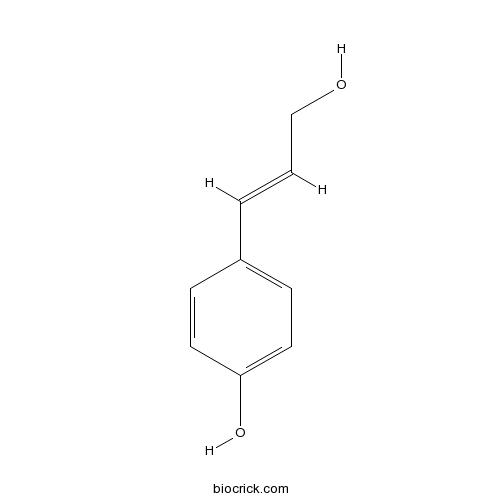
| Formula | C9H10O2 | M.Wt | 150.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | p-Coumaric alcohol; 4-Hydroxycinnamyl alcohol; 3-(p-Hydroxyphenyl) 2-propene 1-ol | ||
| Solubility | Soluble in methan | ||
| Chemical Name | 4-[(E)-3-hydroxyprop-1-enyl]phenol | ||
| SMILES | C1=CC(=CC=C1C=CCO)O | ||
| Standard InChIKey | PTNLHDGQWUGONS-OWOJBTEDSA-N | ||
| Standard InChI | InChI=1S/C9H10O2/c10-7-1-2-8-3-5-9(11)6-4-8/h1-6,10-11H,7H2/b2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. p-Coumaryl alcohol derivatives have antioxidant activity. |

p-Coumaryl alcohol Dilution Calculator

p-Coumaryl alcohol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.6578 mL | 33.2889 mL | 66.5779 mL | 133.1558 mL | 166.4447 mL |
| 5 mM | 1.3316 mL | 6.6578 mL | 13.3156 mL | 26.6312 mL | 33.2889 mL |
| 10 mM | 0.6658 mL | 3.3289 mL | 6.6578 mL | 13.3156 mL | 16.6445 mL |
| 50 mM | 0.1332 mL | 0.6658 mL | 1.3316 mL | 2.6631 mL | 3.3289 mL |
| 100 mM | 0.0666 mL | 0.3329 mL | 0.6658 mL | 1.3316 mL | 1.6644 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- TC 14012
Catalog No.:BCC7910
CAS No.:368874-34-4
- Aucuparin
Catalog No.:BCN7450
CAS No.:3687-28-3
- Tramiprosate
Catalog No.:BCC7727
CAS No.:3687-18-1
- Meclofenoxate hydrochloride
Catalog No.:BCC4170
CAS No.:3685-84-5
- 1,5-Pentanediol diacrylate
Catalog No.:BCC8426
CAS No.:36840-85-4
- Naringenin triacetate
Catalog No.:BCN5425
CAS No.:3682-04-0
- Isohemiphloin
Catalog No.:BCN5424
CAS No.:3682-02-8
- Puerarin
Catalog No.:BCN5958
CAS No.:3681-99-0
- Vitexin
Catalog No.:BCN5423
CAS No.:3681-93-4
- Ribavirin
Catalog No.:BCC4935
CAS No.:36791-04-5
- MRS 2279
Catalog No.:BCC5880
CAS No.:367909-40-8
- 4-Aminophthalimide
Catalog No.:BCC8689
CAS No.:3676-85-5
- Zebularine
Catalog No.:BCC1136
CAS No.:3690-10-6
- 6-epi-Augustifolin
Catalog No.:BCN3233
CAS No.:369390-94-3
- Hydramicromelin B
Catalog No.:BCN7560
CAS No.:369391-55-9
- Icilin
Catalog No.:BCC4074
CAS No.:36945-98-9
- Oroxylin A 7-O-beta-D-glucuronide
Catalog No.:BCN2337
CAS No.:36948-76-2
- TMPyP4 tosylate
Catalog No.:BCC7899
CAS No.:36951-72-1
- FCCP
Catalog No.:BCC5659
CAS No.:370-86-5
- Zinterol hydrochloride
Catalog No.:BCC6911
CAS No.:37000-20-7
- (-)-Variabilin
Catalog No.:BCN4815
CAS No.:370102-93-5
- Carbetocin
Catalog No.:BCC6304
CAS No.:37025-55-1
- Cyclo(L-Phe-L-Pro)
Catalog No.:BCN4029
CAS No.:3705-26-8
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
Combinatorial optimization of synthetic operons for the microbial production of p-coumaryl alcohol with Escherichia coli.[Pubmed:26062542]
Microb Cell Fact. 2015 Jun 11;14:79.
BACKGROUND: Microbes are extensively engineered to produce compounds of biotechnological or pharmaceutical interest. However, functional integration of synthetic pathways into the respective host cell metabolism and optimization of heterologous gene expression for achieving high product titers is still a challenging task. In this manuscript, we describe the optimization of a tetracistronic operon for the microbial production of the plant-derived phenylpropanoid p-Coumaryl alcohol in Escherichia coli. RESULTS: Basis for the construction of a p-Coumaryl alcohol producing strain was the development of Operon-PLICing as method for the rapid combinatorial assembly of synthetic operons. This method is based on the chemical cleavage reaction of phosphorothioate bonds in an iodine/ethanol solution to generate complementary, single-stranded overhangs and subsequent hybridization of multiple DNA-fragments. Furthermore, during the assembly of these DNA-fragments, Operon-PLICing offers the opportunity for balancing gene expression of all pathway genes on the level of translation for maximizing product titers by varying the spacing between the Shine-Dalgarno sequence and START codon. With Operon-PLICing, 81 different clones, each one carrying a different p-Coumaryl alcohol operon, were individually constructed and screened for p-Coumaryl alcohol formation within a few days. The absolute product titer of the best five variants ranged from 48 to 52 mg/L p-Coumaryl alcohol without any further optimization of growth and production conditions. CONCLUSIONS: Operon-PLICing is sequence-independent and thus does not require any specific recognition or target sequences for enzymatic activities since all hybridization sites can be arbitrarily selected. In fact, after PCR-amplification, no endonucleases or ligases, frequently used in other methods, are needed. The modularity, simplicity and robustness of Operon-PLICing would be perfectly suited for an automation of cloning in the microtiter plate format.
AtABCG29 is a monolignol transporter involved in lignin biosynthesis.[Pubmed:22704988]
Curr Biol. 2012 Jul 10;22(13):1207-12.
Lignin is the defining constituent of wood and the second most abundant natural polymer on earth. Lignin is produced by the oxidative coupling of three monolignols: p-Coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol. Monolignols are synthesized via the phenylpropanoid pathway and eventually polymerized in the cell wall by peroxidases and laccases. However, the mechanism whereby monolignols are transported from the cytosol to the cell wall has remained elusive. Here we report the discovery that AtABCG29, an ATP-binding cassette transporter, acts as a p-Coumaryl alcohol transporter. Expression of AtABCG29 promoter-driven reporter genes and a Citrine-AtABCG29 fusion construct revealed that AtABCG29 is targeted to the plasma membrane of the root endodermis and vascular tissue. Moreover, yeasts expressing AtABCG29 exhibited an increased tolerance to p-Coumaryl alcohol by excreting this monolignol. Vesicles isolated from yeasts expressing AtABCG29 exhibited a p-Coumaryl alcohol transport activity. Loss-of-function Arabidopsis mutants contained less lignin subunits and were more sensitive to p-Coumaryl alcohol. Changes in secondary metabolite profiles in abcg29 underline the importance of regulating p-Coumaryl alcohol levels in the cytosol. This is the first identification of a monolignol transporter, closing a crucial gap in our understanding of lignin biosynthesis, which could open new directions for lignin engineering.


