MurrangatinCAS# 37126-91-3 |
- Minumicrolin
Catalog No.:BCN4433
CAS No.:88546-96-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 37126-91-3 | SDF | Download SDF |
| PubChem ID | 181514 | Appearance | Powder |
| Formula | C15H16O5 | M.Wt | 276.3 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
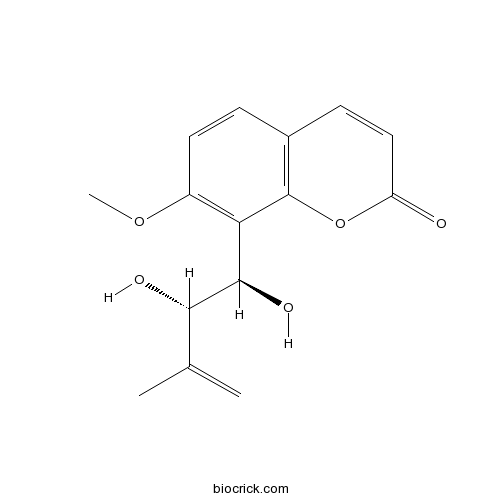
| Chemical Name | 8-[(1R,2S)-1,2-dihydroxy-3-methylbut-3-enyl]-7-methoxychromen-2-one | ||
| SMILES | CC(=C)C(C(C1=C(C=CC2=C1OC(=O)C=C2)OC)O)O | ||
| Standard InChIKey | DKEANOQWICTXTP-UONOGXRCSA-N | ||
| Standard InChI | InChI=1S/C15H16O5/c1-8(2)13(17)14(18)12-10(19-3)6-4-9-5-7-11(16)20-15(9)12/h4-7,13-14,17-18H,1H2,2-3H3/t13-,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Murrangatin and murracarpin show chondroprotective activity by downregulation of interleukin-1β, tumor necrosis factorα, prostaglandins E2,and matrix metalloproteinases -13, and both of them may be a new backbone for developing inhibitors of cyclooxygenase 2. 2. Murrangatin can significantly inhibit Epstein–Barr virus early antigen (EBV–EA) activation, and preserve the high viability of Raji cells, suggests that it may be a valuable anti-tumor-promoting agent. 3. Murrangatin exhibits cytotoxicity against cholangiocarcinoma cell line, KKU-100. 4. Murrangatin exhibits antibacterial activity against P. gingivalis (ATCC 33277). 5. Murrangatin shows soluble epoxide hydrolase inhibitory activity with IC50 values 13.9±6.5uM. |
| Targets | TNF-α | IL Receptor | PGE | COX |

Murrangatin Dilution Calculator

Murrangatin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.6193 mL | 18.0963 mL | 36.1925 mL | 72.3851 mL | 90.4814 mL |
| 5 mM | 0.7239 mL | 3.6193 mL | 7.2385 mL | 14.477 mL | 18.0963 mL |
| 10 mM | 0.3619 mL | 1.8096 mL | 3.6193 mL | 7.2385 mL | 9.0481 mL |
| 50 mM | 0.0724 mL | 0.3619 mL | 0.7239 mL | 1.4477 mL | 1.8096 mL |
| 100 mM | 0.0362 mL | 0.181 mL | 0.3619 mL | 0.7239 mL | 0.9048 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- Azlocillin sodium salt
Catalog No.:BCC4763
CAS No.:37091-65-9
- Procyanidin C1
Catalog No.:BCN6317
CAS No.:37064-30-5
- Z-Glu-OBzl
Catalog No.:BCC2780
CAS No.:3705-42-8
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
- Cyclo(L-Phe-L-Pro)
Catalog No.:BCN4029
CAS No.:3705-26-8
- Carbetocin
Catalog No.:BCC6304
CAS No.:37025-55-1
- (-)-Variabilin
Catalog No.:BCN4815
CAS No.:370102-93-5
- Zinterol hydrochloride
Catalog No.:BCC6911
CAS No.:37000-20-7
- FCCP
Catalog No.:BCC5659
CAS No.:370-86-5
- TMPyP4 tosylate
Catalog No.:BCC7899
CAS No.:36951-72-1
- Oroxylin A 7-O-beta-D-glucuronide
Catalog No.:BCN2337
CAS No.:36948-76-2
- 4'-Amino-3',5'-dichloroacetophenone
Catalog No.:BCC8678
CAS No.:37148-48-4
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- YM201636
Catalog No.:BCC4996
CAS No.:371942-69-7
- ZAPA sulfate
Catalog No.:BCC6563
CAS No.:371962-01-5
- SB 612111 hydrochloride
Catalog No.:BCC7714
CAS No.:371980-94-8
- L-Citruline
Catalog No.:BCN2692
CAS No.:372-75-8
- Capsidiol
Catalog No.:BCC8140
CAS No.:37208-05-2
- Citromycin
Catalog No.:BCN7459
CAS No.:37209-30-6
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- Sieber Linker
Catalog No.:BCC2835
CAS No.:3722-51-8
Coumarins and flavonoid from Murraya paniculata (L.) Jack: Antibacterial and anti-inflammation activity.[Pubmed:26639491]
Pak J Pharm Sci. 2015 Nov;28(6):1947-51.
The ethyl acetate extract of leaves of Murraya paniculata (L.) Jack was described in the previous in vitro study on the inhibition effect on the growth of periodontopathic bacteria and the reduction of cytokines from LPS-stimulated macrophages. In this study, four coumarins including Murrangatin (1), Murrangatin acetate (2), murranganonesenecionate (3), micropubescin (4) and one flavonoid, 3', 4', 5', 7-tetramethoxyflavone (5) were isolated from the leaves of ethyl acetate extract of M. paniculata. MTT assay was used to test cytotoxicity on human gingival fibroblast and monocytes. The isolated compounds were evaluated for their antibacterial effect against Porphyromonas gingivalis (ATCC33277) and anti-inflammation on lipopolysaccharide-stimulated inflammation using monocyte cells. All isolated compounds exhibited antibacterial activity against P. gingivalis (ATCC 33277). Murranganonesenecionate (3) was highly potent anti-inflammation properties. The coumarin constituents from M. paniculata leaves might be potential lead molecules for the development of antimicrobial drugs for treating periodontal disease.
Anti-tumor-promoting effects of 8-substituted 7-methoxycoumarins on Epstein-Barr virus activation assay.[Pubmed:10378778]
Cancer Lett. 1999 Apr 26;138(1-2):87-92.
In a search for anti-tumor-promoting agents, we carried out a primary screening of twenty-nine 8-substituted and four 6-substituted derivatives of 7-methoxycoumarins isolated from plants of the Murraya and/or Citrus species (Rutaceae), examining their possible inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in Raji cells. This investigation indicated that the prenyl (3-methyl-2-butenyl) or 2-hydroxy-3-methylbutyl (or butenyl) unit as an isoprenoid moiety at C-8 on the 7-methoxycoumarin nucleus plays an important role in the anti-tumor-promoting activity. Some of the 8-substituted 7-methoxycoumarins isolated from Murraya species, Murrangatin (7), minumicrolin (10) and chloticol (18), were found to significantly inhibit EBV-EA activation, and preserved the high viability of Raji cells, suggesting that 7, 10 and 18 might be valuable anti-tumor-promoting agents.
C-7 oxygenated coumarins from the fruits of Micromelum minutum.[Pubmed:21544717]
Arch Pharm Res. 2011 Apr;34(4):527-31.
A new 7-oxygenated coumarin, 7-demethylmurralonginol isovalerate (1), and a new natural product, murralonginol (2), together with seven known 7-oxygenated coumarins, murralonginol isovalerate (3), murralongin (4), micromelin (5), scopoletin (6), microminutin (7), Murrangatin (8), and minumicrolin (9), were isolated from the fruits of Micromelum minutum. The structures of these compounds were established on the basis of their 1D and 2D NMR spectroscopic data. Among these isolates, compounds 2 and 4 - 9 exhibited cytotoxicity against cholangiocarcinoma cell line, KKU-100.


