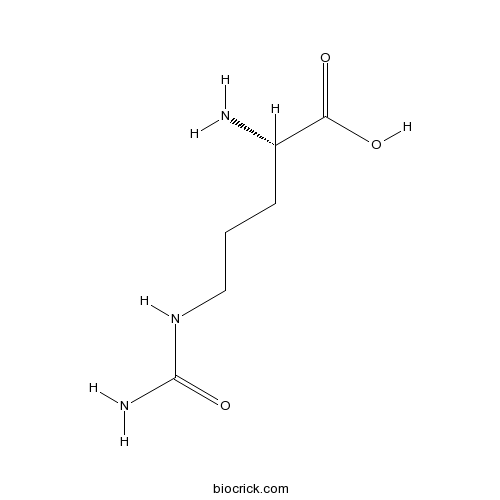
L-CitrulineCAS# 372-75-8 |
- Hydroxyfasudil
Catalog No.:BCC1635
CAS No.:105628-72-6
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- Hydroxyfasudil hydrochloride
Catalog No.:BCC1636
CAS No.:155558-32-0
- H-1152 dihydrochloride
Catalog No.:BCC1616
CAS No.:871543-07-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 372-75-8 | SDF | Download SDF |
| PubChem ID | 9750 | Appearance | Powder |
| Formula | C6H13N3O3 | M.Wt | 175.18 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | H2O : ≥ 50 mg/mL (285.40 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (2S)-2-amino-5-(carbamoylamino)pentanoic acid | ||
| SMILES | C(CC(C(=O)O)N)CNC(=O)N | ||
| Standard InChIKey | RHGKLRLOHDJJDR-BYPYZUCNSA-N | ||
| Standard InChI | InChI=1S/C6H13N3O3/c7-4(5(10)11)2-1-3-9-6(8)12/h4H,1-3,7H2,(H,10,11)(H3,8,9,12)/t4-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | L-citrulline is a substance called a non-essential amino acid that is used as a sports performance and cardiovascular health supplement. |
| Targets | NO | IL Receptor |
| In vivo | Digital Plethysmography and Arginine Metabolism in Prehypertension—Effect of Nebivolol Therapy[Reference: WebLink]The Journal of Clinical Hypertension Volume 17, Issue 1, pages 14–19, January 2015Prehypertension is an important phenotype for cardiovascular risk and development of established hypertension. l-Citrulline Protects from Kidney Damage in Type 1 Diabetic Mice.[Pubmed: 24400007]Front Immunol. 2013 Dec 24;4:480.Diabetic nephropathy (DN) is a major cause of end-stage renal disease, associated with endothelial dysfunction. Chronic supplementation of l-arginine (l-arg), the substrate for endothelial nitric oxide synthase (eNOS), failed to improve vascular function. L-Citruline (l-cit) supplementation not only increases l-arg synthesis, but also inhibits cytosolic arginase I, a competitor of eNOS for the use of l-arg, in the vasculature.
To investigate whether l-cit treatment reduces DN in streptozotocin (STZ)-induced type 1 diabetes (T1D) in mice and rats and to study its effects on arginase II (ArgII) function, the main renal isoform.
|

L-Citruline Dilution Calculator

L-Citruline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.7084 mL | 28.5421 mL | 57.0841 mL | 114.1683 mL | 142.7104 mL |
| 5 mM | 1.1417 mL | 5.7084 mL | 11.4168 mL | 22.8337 mL | 28.5421 mL |
| 10 mM | 0.5708 mL | 2.8542 mL | 5.7084 mL | 11.4168 mL | 14.271 mL |
| 50 mM | 0.1142 mL | 0.5708 mL | 1.1417 mL | 2.2834 mL | 2.8542 mL |
| 100 mM | 0.0571 mL | 0.2854 mL | 0.5708 mL | 1.1417 mL | 1.4271 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
H-Cit-OH
- SB 612111 hydrochloride
Catalog No.:BCC7714
CAS No.:371980-94-8
- ZAPA sulfate
Catalog No.:BCC6563
CAS No.:371962-01-5
- YM201636
Catalog No.:BCC4996
CAS No.:371942-69-7
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- 4'-Amino-3',5'-dichloroacetophenone
Catalog No.:BCC8678
CAS No.:37148-48-4
- Murrangatin
Catalog No.:BCN5426
CAS No.:37126-91-3
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- Azlocillin sodium salt
Catalog No.:BCC4763
CAS No.:37091-65-9
- Procyanidin C1
Catalog No.:BCN6317
CAS No.:37064-30-5
- Z-Glu-OBzl
Catalog No.:BCC2780
CAS No.:3705-42-8
- Capsidiol
Catalog No.:BCC8140
CAS No.:37208-05-2
- Citromycin
Catalog No.:BCN7459
CAS No.:37209-30-6
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- Sieber Linker
Catalog No.:BCC2835
CAS No.:3722-51-8
- Wilforgine
Catalog No.:BCN5427
CAS No.:37239-47-7
- Wilfortrine
Catalog No.:BCN3085
CAS No.:37239-48-8
- Wilfordine
Catalog No.:BCN3083
CAS No.:37239-51-3
- TCS OX2 29
Catalog No.:BCC7670
CAS No.:372523-75-6
- Sennoside C
Catalog No.:BCN1004
CAS No.:37271-16-2
- Sennoside D
Catalog No.:BCN1005
CAS No.:37271-17-3
- H-D-Tyr-OMe.HCl
Catalog No.:BCC3135
CAS No.:3728-20-9
- Flavokawain C
Catalog No.:BCN8456
CAS No.:37308-75-1
Effectiveness of arginase inhibitors against experimentally induced stroke.[Pubmed:29600431]
Naunyn Schmiedebergs Arch Pharmacol. 2018 Jun;391(6):603-612.
Stroke is a lethal disease, but it disables more than it kills. Stroke is the second leading cause of death and the most frequent cause of permanent disability in adults worldwide, with 90% of survivors having residual deficits. The pathophysiology of stroke is complex and involves a strong inflammatory response associated with oxidative stress and activation of several proteolytic enzymes. The current study was designed to investigate the effect of arginase inhibitors (L-Citruline and L-ornithine) against ischemic stroke induced in rats by middle cerebral artery occlusion (MCAO). MCAO resulted in alteration in rat behavior, brain infarct, and edema associated with disruption of the blood-brain barrier (BBB). This was mediated through overexpression of arginase I and II, inducible NOS (iNOS), malondialdehyde (MDA), advanced glycation end products (AGEs), TNF-alpha, and IL-1beta and downregulation of endothelial nitric oxide synthase (eNOS). Treatment with L-Citruline and L-ornithine and the standard neuroprotective drug cerebrolysin ameliorated all the deleterious effects of stroke. These results indicate the possible use of arginase inhibitors in the treatment of stroke after suitable clinical trials are done.
Digital plethysmography and arginine metabolism in prehypertension: effect of nebivolol therapy.[Pubmed:25495953]
J Clin Hypertens (Greenwich). 2015 Jan;17(1):14-9.
Prehypertension is an important phenotype for cardiovascular risk and development of established hypertension. To better understand the early circulatory changes in this group, the authors studied 34 patients with prehypertension (blood pressure 120-139/80-89 mm Hg) using digital plethysmography for measurement of blood flow and reactive hyperemic index (RHI). Arterial augmentation index (AI) was also measured. Because prehypertension is associated with endothelial dysfunction and decreased availability of nitric oxide (NO), indices of arginine metabolism (l-arginine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine, and l-citrulline) were measured. Nebivolol (5 mg/d), a vasodilating beta1 -antagonist with beta3 -agonist activity, was studied in a double-blind fashion for 8 weeks. Nebivolol increases the bioavailability of NO. Prehypertension was associated with normal RHI and baseline digital blood flow. AI was abnormal and associated with diastolic blood pressure. ADMA concentration was increased at baseline. After 8 weeks of nebivolol therapy, RHI, ADMA, symmetric dimethylarginine, and AI showed no significant change, but digital blood flow and l-citrulline levels were significantly increased. Prehypertension is associated with increased ADMA and evidence of increased arterial stiffness and preserved RHI. Nebivolol therapy is associated with digital vasodilation and increased NO production, as depicted by increased levels of L-Citruline and mean digital blood flow.
Oral L-arginine supplementation in patients with mild arterial hypertension and its effect on plasma level of asymmetric dimethylarginine, L-citruline, L-arginine and antioxidant status.[Pubmed:23161038]
Eur Rev Med Pharmacol Sci. 2012 Nov;16(12):1665-74.
BACKGROUND: Potential role of L-arginine supplementation as a new effective strategy of improving endothelial function in patients with hypertension is recently under consideration. OBJECTIVE: To evaluate influence of 28-day oral supplementation of L-arginine on plasma level of asymmetric dimethylarginine (ADMA), L-citrulline, L-arginine and total antioxidant status (TAS), in patients with mild arterial hypertension. SUBJECTS AND METHODS: 54 participants (24 women and 30 men) were studied. Ambulatory blood pressure monitoring (ABPM) was used for allotting patients to either healthy control group (19 subjects) or hypertensive treatment group (35 patients). Patients were later randomized to either L-arginine (2 g tid or 4 g tid) or placebo. During 28 days of study on 5 consecutive visits TAS, plasma level of ADMA, L-citrulline, and L-arginine were measured. RESULTS: In patients with mild hypertension treated with L-arginine significant increase in TAS and plasma level of arginine and citrulline was observed. Additionally plasma ADMA concentrations after 28 days of L-arginine supplementation significantly exceeded initial concentrations. CONCLUSIONS: L-arginine supplementation increases plasma arginine, citrulline and TAS in patients with mild arterial hypertension. It confirms the thesis that augmented concentrations of L-arginine stimulate NO biosynthesis which leads to reduction of oxidative stress. Increase of ADMA plasma level after L-arginine supplementation confirms correlation between ADMA and L-arginine.
L-arginine transport and nitric oxide synthesis in human endothelial progenitor cells.[Pubmed:23143655]
J Cardiovasc Pharmacol. 2012 Nov;60(5):439-49.
Nitric oxide (NO) is an endogenous vasodilator molecule synthetized from L-arginine by a family of nitric oxide synthases. In differentiated human endothelial cells, it is well known that L-arginine uptake via cationic amino acid transporters (y(+)/CAT) or system y(+)L is required for the NO synthesis via endothelial nitric oxide synthase, but there are no reports in human endothelial progenitor cell (hEPC). Therefore, we isolated hEPCs from peripheral blood of healthy donors and cultured them for either 3 (hEPC-3d) or 14 days (hEPC-14d) to characterize the L-arginine transport and NO synthesis in those cells. L-arginine transport and NO synthesis were analyzed in the presence or absence of N-ethylmaleimide or L-nitroarginine methyl ester, as inhibitors of y(+)/CAT system and nitric oxide synthases, respectively. The results showed that L-arginine uptake is higher in hEPC-14d than in hEPC-3d. Kinetic parameters for L-arginine transport showed the existence of at least 2 transporter systems in hEPC: a high affinity transporter system (K(m)= 4.8 +/- 1.1 muM for hEPC-3d and 6.1 +/- 2.4 muM for hEPC-14d) and a medium affinity transporter system (K(m) = 85.1 +/- 4.0 muM for hEPC-3d and 95.1 +/- 8 muM for hEPC-14d). Accordingly, hEPC expressed mRNA and protein for CAT-1 (ie, system y(+)) and mRNA for 2 subunits of y(+)L system, yLAT1, and 4F2hc. Higher L-Citruline production and NO bioavailability (4-fold), and endothelial nitric oxide synthase expression (both mRNA and protein) were observed in hEPC-14d compared with hEPC-3d. Finally, the high L-Citruline formation observed in hEPC-14d was blocked by N-ethylmaleimide. In conclusion, this study allowed to identity a functional L-arginine/NO pathway in two hEPC differentiation stages, which improves the understanding of the physiology of these precursor cells.
Polymorphisms of the NOS3 gene in Southern Chilean subjects with coronary artery disease and controls.[Pubmed:19931521]
Clin Chim Acta. 2010 Feb;411(3-4):258-62.
BACKGROUND: Nitric oxide (NO) from the endothelium, produced by oxidation of l-arginine to L-Citruline for the action at the endothelial nitric oxide synthase (eNOS) is considered an important atheroprotective factor. The 894G>T, -786T>C and 4a/4b polymorphic variants of the NOS3 gene have been implicated in the development of coronary artery disease (CAD). We investigated the association between occurrence of CAD documented by angiography and the 894G>T, -786T>C and 4a/4b polymorphisms of the NOS3 gene in Southern Chilean individuals. METHODS: A total of 112 unrelated patients with diagnosis of CAD confirmed by angiography and 112 controls were included in this study. The 894G>T and -786T>C single nucleotide polymorphisms were analyzed by PCR-RFLP, and 4a/4b polymorphism just for PCR. RESULTS: The genotype distribution and the relative allelic frequencies for the 3 variants investigated were not significantly different between CAD and control subjects (p=NS). Moreover, the odds ratio for CAD associated with the 894T (OR=1.22, 95% CI 0.76-1.95), -786C (OR=1.16, 95% CI 0.75-1.80) and 4a (OR=0.97, 95% CI 0.48-1.95) variants failed to reach statistical significance. CONCLUSION: These findings suggest that the 894G>T, -786T>C and 4a/4b polymorphisms of the NOS3 were not associated with CAD in the studied subjects.
Endothelial nitric oxide synthase G894T gene polymorphism in Chilean subjects with coronary artery disease and controls.[Pubmed:16616056]
Clin Chim Acta. 2006 Sep;371(1-2):102-6.
BACKGROUND: Nitric oxide (NO) from the endothelium, produced by oxidation of l-arginine to L-Citruline for the action at the endothelial nitric oxide synthase (eNOS), is considered an important atheroprotective factor. The Glu298Asp (G894T) polymorphic variant of the eNOS gene has been implicated in the development of coronary artery disease (CAD). We investigated the association between occurrence of CAD documented by angiography and the G894T polymorphism of the NOS3 gene in Chilean individuals. METHODS: A total of 112 unrelated patients with diagnosis of CAD and 72 controls were included in this study. G894T gene polymorphism was analyzed by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP). RESULTS: The frequency of TT homozygous genotype for G894T polymorphism was 7% in CAD patients and 1% in the control group. However, the genotype distribution and allele frequencies were not significantly different between CAD and control subjects (P>0.05). Moreover, the odds ratio for CAD associated with the T variant failed to reach statistical significance (OR=1.5; 95% CI: 0.87-2.59, P>0.05). CONCLUSION: These findings suggest that the G894T polymorphism of the eNOS gene was not associated with CAD in Chilean individuals.
Calcitonin gene-related peptide-enhanced nitric oxide release and inducible NOS activity and mRNA expression in LPS-stimulated mouse peritoneal macrophages.[Pubmed:11442318]
Shock. 2001 Jul;16(1):64-9.
Previously we have shown that calcitonin gene-related peptide (CGRP), a neuropeptide increases lipopolysaccharide- (LPS) induced nitric oxide (NO) production in mouse peritoneal macrophages by using the Griess method. In this study we further examined whether CGRP could modulate inducible NO synthase (iNOS) protein and mRNA expression from mouse peritoneal macrophages. Macrophages were obtained from the peritoneal exude of male Balb/c mouse. The cells were plated on culture dishes at a density of 5 x 10(5) cells per well and were allowed to adhere for 2 h. After incubation for 24 h, the macrophages were cultured with 0.01 to 1 microg/mL LPS with or without CGRP (1-1,000 nM) for 24 h. The results showed that CGRP markedly enhanced 0.5 microg/mL LPS-induced NO release as compared with that of lower doses of LPS, such as 0.01 and 0.1 microg/mL LPS. NO was increased from 19.8 +/- 2.6 to the highest level of 31.5 +/- 4.2 microM in 5 x 10(5) cells by 10 nM CGRP in 0.5 microg/mL LPS-stimulated macrophages. The cGMP level in macrophages was augmented when CGRP was added with LPS. However, when using higher dose (1.0 microg/mL) of LPS to stimulate the macrophages, CGRP had no effect at all on NO release. CGRP had no direct effect on NO and cGMP production. CGRP increased the expression of inducible NOS protein in LPS-stimulated macrophages shown by immunocytochemistry method. The activity of iNOS was also enhanced by CGRP as compared with LPS-stimulation alone by detecting the 3H-L-Citruline formation from 3H-L-arginine. We found that CGRP also increased the LPS-induced iNOS mRNA levels by using reverse transcriptase-PCR method. These data suggest that CGRP enhances LPS-induced NO release, iNOS activity, and iNOS mRNA in mouse peritoneal macrophages.
[Nitric oxide as a regulator of hemodynamic changes in pregnancy].[Pubmed:10085607]
Ginecol Obstet Mex. 1999 Jan;67:29-36.
Nitric oxide (.NO) produced by the majority of animal cells, has been considered a second messenger, since it is the result of a transduction process induced by a first stimulus. Biochemically, .NO is produced during the conversion of L-arginine to L-Citruline by a reaction catalyzed by the enzyme nitric oxide synthetase. Two ixoenzymes have been characterized from this enzyme: a constitutive isoenzyme activated by hormones produced by the endothelial cells and acting on smooth muscle relaxing properties and the other, an inducible isoenzyme whose synthesis is stimulated by cytokines, and produced by macrophages. As pregnancy progresses, the concentrations of .NO, its metabolites, nitrates and nitrites, cGMP and the synthesizing enzyme, nitric oxide synthetase, increase parallelly until reaching a maximum peak before birth. It is considered that .NO is the molecule that maintains the typical vasodilated tone during pregnancy. During preeclampsia, this free radical, as well as its metabolites are found to be significantly decreased, in addition, the administration of .NO donors or of the precursor of L-arginine reverts the vascular abnormalities of this condition. The mechanism of action behind .NO on the vascular endothelium is by its stimulating effect on the enzyme cyclase guanilate, causing an increase in cGMP concentration and the relaxation of the smooth muscle. The nitric oxide generates by macrophages acts as a defense mechanism when linked with other radicals as the superoxide anion (O2).
[Role of endothelial nitric oxide synthases in the contractile response to angiotensin II of the aorta in rats. Wistar Kyoto and hypertensive rats].[Pubmed:7538750]
Arch Mal Coeur Vaiss. 1994 Aug;87(8):1001-4.
Dysfunctions of EDRF/L-arginine-NO pathway have been demonstrated in genetic and experimental hypertension. NO is produced through the conversion of L-arginine to L-Citruline by NO synthases (NOS) which exist at least in two isoforms. The first termed constitutive (NOSc) and located in the endothelium of the vascular wall results in the basal and stimulated NO production. The second termed inducible (NOSi), which produces large amounts of NO, can be expressed in both smooth muscle and endothelial cells. The aim of the study was to examine and compare in isolated aortic rings of WKY and SHR rats, the activity of the two isoforms of endothelial NO synthases and their influence on the constrictor response induced by angiotensin II. On phenylephrine preconstricted endothelium intact aortic rings (10(-6) M, WKY = 1.2 +/- 0.04 g; SHR = 1.2 +/- 0.07 g; n = 7), carbachol (10(-5) M) induced a greater relaxation in WKY (84 +/- 2.5%) than in SHR (63 +/- 8.5%) rat. This suggests the presence of a low NOSc stimulated activity in the hypertensive strain. When the incubation period was limited to 30 min after equilibration period, L-arginine (3.10(-4) M) did not induce relaxation. When the incubation period was prolonged (180 min), L-arginine induced a relaxation (WKY = 75 +/- 8%; SHR = 58 +/- 10%; n = 7). This relaxation was not observed in a medium containing actinomycin D (10(-6) M) or after endothelium removal, indicating the induction of an endothelial NOSi in the two strains.(ABSTRACT TRUNCATED AT 250 WORDS)
[An enteral modular formula in dibasic amino aciduria].[Pubmed:8011796]
Nutr Hosp. 1993 Sep-Oct;8(7):441-6.
Lysinuric protein intolerance (LPI) or dibasic amino acid aminoaciduria is an unusual metabolic illness which, in countries where it is most common, affects one individual for every 60,000-80,000 births, and which is characterised by the inability to transport dibasic amino acids to the interior of the different cells of the organism. This paper sets out the design for an enteral modular formula for a pediatric patient very probably suffering from LPI. The formula has a very limited protein content, and incorporates L-Citruline, a non-proteinogenic amino acid which intervenes in the urea cycle.
Effects of guanidino compounds on the endothelium-derived relaxing factor inhibitor NG-monomethyl L-arginine.[Pubmed:1719191]
J Pharmacol Exp Ther. 1991 Nov;259(2):490-4.
The vasoconstrictor effect of NG-monomethyl L-arginine (L-NMMA) is believed to be due to the inhibition of the synthesis of endothelium-derived relaxing factor (EDRF) from L-arginine. Here, we tested a series of guanidino compounds other than L-arginine on the rat aorta preparation with and without endothelium present. None of the compounds promoted vascular relaxation like N alpha-benzoyl L-arginine ethyl ester or elicited vasoconstriction like L-NMMA. We discovered that the two guanidino compounds, L-homoarginine (L-HA) and L-amino-tau-guanidino butyric acid (L-AGBA), behave like L-arginine and reversed the vasoconstrictor effect of L-NMMA. This effect was stereospecific and concentration-dependent. The order of potency to overcome the effects of L-NMMA was L-HA, followed by L-AGBA. This was the same order of potency for overcoming the inhibitory effects of L-NMMA on cyclic GMP formation. Two related compounds, L-amino guanidino propionic acid and guanidine, were ineffective. Furthermore, high performance liquid chromatography analysis showed that rat aortic vessels contain the same amount of L-arginine in the presence or absence of endothelium, and no detectable amount of L-Citruline is formed during endothelium-dependent relaxation. We conclude that the reversal of the effects of L-NMMA by L-HA and L-AGBA is due to their structural similarities to L-NMMA and not to synthesis of an EDRF-like material from these guanidines. We suggest that some of the inhibition of L-NMMA by L-arginine may have a similar basis of structural antagonism.


