WilforgineCAS# 37239-47-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 37239-47-7 | SDF | Download SDF |
| PubChem ID | 124030 | Appearance | White powder |
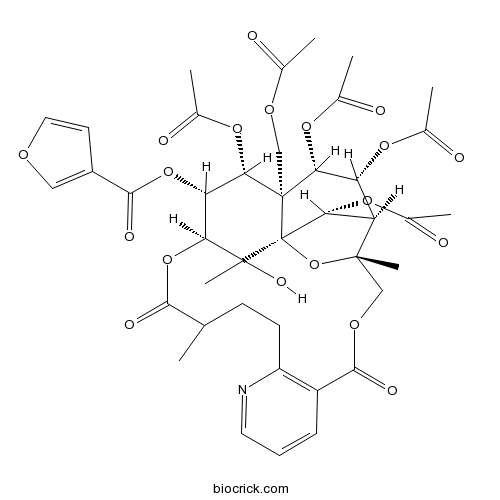
| Formula | C41H47NO19 | M.Wt | 857.8 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in methanol; insoluble in water | ||
| SMILES | CC1CCC2=C(C=CC=N2)C(=O)OCC3(C4C(C(C5(C(C(C(C(C5(C4OC(=O)C)O3)(C)O)OC1=O)OC(=O)C6=COC=C6)OC(=O)C)COC(=O)C)OC(=O)C)OC(=O)C)C | ||
| Standard InChIKey | QFIYSPKZWOALMZ-YHQLYFKISA-N | ||
| Standard InChI | InChI=1S/C41H47NO19/c1-19-11-12-27-26(10-9-14-42-27)37(50)54-17-38(7)28-29(55-21(3)44)33(57-23(5)46)40(18-53-20(2)43)34(58-24(6)47)30(59-36(49)25-13-15-52-16-25)32(60-35(19)48)39(8,51)41(40,61-38)31(28)56-22(4)45/h9-10,13-16,19,28-34,51H,11-12,17-18H2,1-8H3/t19?,28-,29-,30+,31-,32+,33-,34+,38+,39?,40-,41+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Wilforgine exhibits insecticidal activity of some degree. |
| In vitro | Effects of plant stress signal molecules on the production of wilforgine in an endophytic actinomycete isolated from Tripterygium wilfordii Hook.f.[Pubmed: 25523369]Curr Microbiol. 2015 Apr;70(4):571-9.The endophytic actinomycete F4-20 was isolated from Tripterygium wilfordii Hook.f. and was confirmed to produce Wilforgine, a secondary metabolite discovered in its host. Insecticidal activities and active ingredients of Tripterygium hypoglaucum (Levl.) Hutch[Reference: WebLink]Acta Entomologica Sinica,2007,50(8):795-800.Isolating insecticidal activities compounds is an important method to discover new pesticides. |
| Structure Identification | Zhongguo Zhong Yao Za Zhi. 2014 Jun;39(12):2267-74.Effects of amino acid on growth and secondary metabolites contents of adventitious roots of Tripterygium wilfordii.[Pubmed: 25244757]The adventitious root of Tripterygium wilfordii was used as experiment material to study effects of various concentration of aspartic acid, isoleucine, cysteine and arginine in MS medium on the growth and triptolide, Wilforgine, wilforine contents of the adventitious roots. |

Wilforgine Dilution Calculator

Wilforgine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1658 mL | 5.8289 mL | 11.6577 mL | 23.3155 mL | 29.1443 mL |
| 5 mM | 0.2332 mL | 1.1658 mL | 2.3315 mL | 4.6631 mL | 5.8289 mL |
| 10 mM | 0.1166 mL | 0.5829 mL | 1.1658 mL | 2.3315 mL | 2.9144 mL |
| 50 mM | 0.0233 mL | 0.1166 mL | 0.2332 mL | 0.4663 mL | 0.5829 mL |
| 100 mM | 0.0117 mL | 0.0583 mL | 0.1166 mL | 0.2332 mL | 0.2914 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sieber Linker
Catalog No.:BCC2835
CAS No.:3722-51-8
- PIK-75
Catalog No.:BCC1163
CAS No.:372196-77-5
- Citromycin
Catalog No.:BCN7459
CAS No.:37209-30-6
- Capsidiol
Catalog No.:BCC8140
CAS No.:37208-05-2
- L-Citruline
Catalog No.:BCN2692
CAS No.:372-75-8
- SB 612111 hydrochloride
Catalog No.:BCC7714
CAS No.:371980-94-8
- ZAPA sulfate
Catalog No.:BCC6563
CAS No.:371962-01-5
- YM201636
Catalog No.:BCC4996
CAS No.:371942-69-7
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- 4'-Amino-3',5'-dichloroacetophenone
Catalog No.:BCC8678
CAS No.:37148-48-4
- Wilfortrine
Catalog No.:BCN3085
CAS No.:37239-48-8
- Wilfordine
Catalog No.:BCN3083
CAS No.:37239-51-3
- TCS OX2 29
Catalog No.:BCC7670
CAS No.:372523-75-6
- Sennoside C
Catalog No.:BCN1004
CAS No.:37271-16-2
- Sennoside D
Catalog No.:BCN1005
CAS No.:37271-17-3
- H-D-Tyr-OMe.HCl
Catalog No.:BCC3135
CAS No.:3728-20-9
- Flavokawain C
Catalog No.:BCN8456
CAS No.:37308-75-1
- 3-Quinuclidinone
Catalog No.:BCC8642
CAS No.:3731-38-2
- Decloxizine
Catalog No.:BCC5529
CAS No.:3733-63-9
- Cephaeline Hydrochloride
Catalog No.:BCC8307
CAS No.:3738-70-3
- DS2
Catalog No.:BCC7748
CAS No.:374084-31-8
- Boc-DL-Ala-OH
Catalog No.:BCC3050
CAS No.:3744-87-4
[Effects of amino acid on growth and secondary metabolites contents of adventitious roots of Tripterygium wilfordii].[Pubmed:25244757]
Zhongguo Zhong Yao Za Zhi. 2014 Jun;39(12):2267-74.
The adventitious root of Tripterygium wilfordii was used as experiment material to study effects of various concentration of aspartic acid, isoleucine, cysteine and arginine in MS medium on the growth and triptolide, Wilforgine, wilforine contents of the adventitious roots. The results showed that compared with the control, supplemented with 0.25 mmol x L(-1) aspartic acid at 3rd week, the growth of the adventitious roots only accounted for 80%, but the content of triptolide of the adventitious roots and the medium was 1.36, 1.30 times, the content of Wilforgine was 1.16, 1.37 times, the content of wilforine was 1.22, 1.63 times, respectively. At 3rd week 0.05 mmol x L(-1) isoleucine, the growth of adventitious roots was 97.3%, Wilforgine of adventitious roots and medium 1.02, 1.27 times, wilforine 1.36 times and 1.15 times. At 1st week 0.25 mmol x L(-1) cysteine, the growth of the adventitious roots comprised 77.5% of the control, while content of triptolide of adventitious roots reached 1.87 times. At 2nd week 1.00 mmol x L(-1) cysteine, the growth of adventitious roots was 44.6% of the control, the content of wilforine in medium was 2.97 times. At 3rd week 0.50 mmol x L(-1) arginine, the growth of adventitious roots was 124.2%, the content of Wilforgine and wilforine was 1.3, 1.4 times, respectively.
Effects of plant stress signal molecules on the production of wilforgine in an endophytic actinomycete isolated from Tripterygium wilfordii Hook.f.[Pubmed:25523369]
Curr Microbiol. 2015 Apr;70(4):571-9.
The endophytic actinomycete F4-20 was isolated from Tripterygium wilfordii Hook.f. and was confirmed to produce Wilforgine, a secondary metabolite discovered in its host. F4-20 showed a close phylogenetic relationship to Streptomyces species. To seek elicitors that may enhance the production of Wilforgine in F4-20, four plant stress molecules were applied to the in vitro liquid cultures. Results showed that methyl jasmonate (MeJA), salicylic acid (SA), and hydrogen peroxide (H2O2) inhibited bacterial growth, whereas glutathione (GSH) treatment significantly increased bacterial growth. The Wilforgine contents in the mycelia of F4-20 were reduced by MeJA and GSH but were induced by SA and H2O2. When added in the end of the culture period (7 day), 1 mM SA and 5 mM H2O2 resulted in 69.35 +/- 1.71 and 71.80 +/- 3.35 microg/g DW of Wilforgine production, 1.55 and 1.60 fold to that of control (44.83 +/- 1.35 microg/g DW), respectively. Though this improved production was about 6.5 times lower than that of the natural root (454.00 microg/g dry root bark), it provided an alternative method for the production of valuable plant secondary metabolites.


