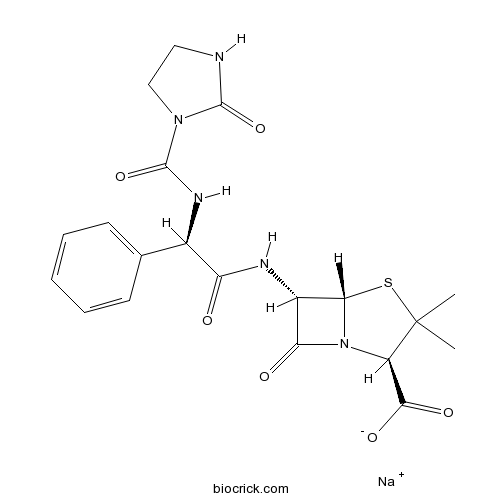
Azlocillin sodium saltCAS# 37091-65-9 |
- Nateglinide
Catalog No.:BCC5005
CAS No.:105816-04-4
- ML133 HCl
Catalog No.:BCC5006
CAS No.:1222781-70-5
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- Gliclazide
Catalog No.:BCC5002
CAS No.:21187-98-4
- Tolbutamide
Catalog No.:BCC5001
CAS No.:64-77-7
- Nicorandil
Catalog No.:BCC5004
CAS No.:65141-46-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 37091-65-9 | SDF | Download SDF |
| PubChem ID | 23681186 | Appearance | Powder |
| Formula | C20H22N5NaO6S | M.Wt | 483.5 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Sodium azlocillin | ||
| Solubility | DMSO : 100 mg/mL (206.84 mM; Need ultrasonic) H2O : 6.67 mg/mL (13.80 mM; Need ultrasonic) | ||
| Chemical Name | sodium;(2R,5S,6S)-3,3-dimethyl-7-oxo-6-[[(2R)-2-[(2-oxoimidazolidine-1-carbonyl)amino]-2-phenylacetyl]amino]-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate | ||
| SMILES | CC1(C(N2C(S1)C(C2=O)NC(=O)C(C3=CC=CC=C3)NC(=O)N4CCNC4=O)C(=O)[O-])C.[Na+] | ||
| Standard InChIKey | UVOCNBWUHNCKJM-QXKADHPISA-M | ||
| Standard InChI | InChI=1S/C20H23N5O6S.Na/c1-20(2)13(17(28)29)25-15(27)12(16(25)32-20)22-14(26)11(10-6-4-3-5-7-10)23-19(31)24-9-8-21-18(24)30;/h3-7,11-13,16H,8-9H2,1-2H3,(H,21,30)(H,22,26)(H,23,31)(H,28,29);/q;+1/p-1/t11-,12+,13-,16+;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Azlocillin is an acylampicillin with a broad spectrum against bacteria.
Target: Antibacterial
Azlocillin (12.5 μg/mL) inhibits over 75% of the isolates of Pseudomonas aeruginosa. Azlocillin (12.5 μg/mL) is also active against indole-negative and -positive Proteus spp., inhibiting 98 and 71%, respectively. Azlocillin is more active than mezlocillin, ticarcillin, and carbenicillin and as active as BLP-1654 against isolates of P. aeruginosa [1]. The acyl side chains of Azlocillin have an ureido-(urea) structurehence the name ureidopenicillins or, more specifically, acylureidopenicillins. In vitro studies against P. aeruginosa demonstrates that piperacillin has activity that is twice that of azlocillin, 4 times that of mezlocillin and ticarcillin, and about 8 times that of carbenicillin. Azlocillin produces elongated bacterial forms with delayed or no lysis in morphologic studies [2].
Azlocillin has MICs of 12.5 μg/mL on Pseudomonas aeruginosa. Azlocillin (3.125 μg/mL) results in a reduction in the rate of growth but no bactericidal phase on Pseudomonas aeruginosa. Azlocillin decreases an initial lag phase with increasing drug concentration. At the lower concentration of tobramycin (0.5 μg/ml), the combinations with both the high and the low concentrations of Azlocillin are more effective than the individual components on Pseudomonas aeruginosa [3]. Isolates with derepression of AmpC enzyme are one to two doubling dilutions more resistant to azlocillin than are those in which increased efflux or impermeability is inferred. Those with secondary β-lactamases are mostly (12/14 cases) susceptible to ceftazidime at 4 mg/L, but are amongst the most resistant to Azlocillin (MIC ≥128 mg/L in 10/14 cases) [4]. References: | |||||

Azlocillin sodium salt Dilution Calculator

Azlocillin sodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.0683 mL | 10.3413 mL | 20.6825 mL | 41.365 mL | 51.7063 mL |
| 5 mM | 0.4137 mL | 2.0683 mL | 4.1365 mL | 8.273 mL | 10.3413 mL |
| 10 mM | 0.2068 mL | 1.0341 mL | 2.0683 mL | 4.1365 mL | 5.1706 mL |
| 50 mM | 0.0414 mL | 0.2068 mL | 0.4137 mL | 0.8273 mL | 1.0341 mL |
| 100 mM | 0.0207 mL | 0.1034 mL | 0.2068 mL | 0.4137 mL | 0.5171 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Azlocillin is an acylampicillin with a broad spectrum against bacteria.
- Procyanidin C1
Catalog No.:BCN6317
CAS No.:37064-30-5
- Z-Glu-OBzl
Catalog No.:BCC2780
CAS No.:3705-42-8
- Cyclo(Gly-L-Pro)
Catalog No.:BCN4059
CAS No.:3705-27-9
- Cyclo(L-Phe-L-Pro)
Catalog No.:BCN4029
CAS No.:3705-26-8
- Carbetocin
Catalog No.:BCC6304
CAS No.:37025-55-1
- (-)-Variabilin
Catalog No.:BCN4815
CAS No.:370102-93-5
- Zinterol hydrochloride
Catalog No.:BCC6911
CAS No.:37000-20-7
- FCCP
Catalog No.:BCC5659
CAS No.:370-86-5
- TMPyP4 tosylate
Catalog No.:BCC7899
CAS No.:36951-72-1
- Oroxylin A 7-O-beta-D-glucuronide
Catalog No.:BCN2337
CAS No.:36948-76-2
- Icilin
Catalog No.:BCC4074
CAS No.:36945-98-9
- Hydramicromelin B
Catalog No.:BCN7560
CAS No.:369391-55-9
- IC-87114
Catalog No.:BCC1161
CAS No.:371242-69-2
- Murrangatin
Catalog No.:BCN5426
CAS No.:37126-91-3
- 4'-Amino-3',5'-dichloroacetophenone
Catalog No.:BCC8678
CAS No.:37148-48-4
- Flavoxate hydrochloride
Catalog No.:BCC5208
CAS No.:3717-88-2
- PI-103
Catalog No.:BCC1162
CAS No.:371935-74-9
- PI-103 Hydrochloride
Catalog No.:BCC1860
CAS No.:371935-79-4
- YM201636
Catalog No.:BCC4996
CAS No.:371942-69-7
- ZAPA sulfate
Catalog No.:BCC6563
CAS No.:371962-01-5
- SB 612111 hydrochloride
Catalog No.:BCC7714
CAS No.:371980-94-8
- L-Citruline
Catalog No.:BCN2692
CAS No.:372-75-8
- Capsidiol
Catalog No.:BCC8140
CAS No.:37208-05-2
- Citromycin
Catalog No.:BCN7459
CAS No.:37209-30-6
Comparison of methods for processing drinking water samples for the isolation of Mycobacterium avium and Mycobacterium intracellulare.[Pubmed:18359837]
Appl Environ Microbiol. 2008 May;74(10):3094-8.
Several protocols for isolation of mycobacteria from water exist, but there is no established standard method. This study compared methods of processing potable water samples for the isolation of Mycobacterium avium and Mycobacterium intracellulare using spiked sterilized water and tap water decontaminated using 0.005% cetylpyridinium chloride (CPC). Samples were concentrated by centrifugation or filtration and inoculated onto Middlebrook 7H10 and 7H11 plates and Lowenstein-Jensen slants and into mycobacterial growth indicator tubes with or without polymyxin, azlocillin, nalidixic acid, trimethoprim, and amphotericin B. The solid media were incubated at 32 degrees C, at 35 degrees C, and at 35 degrees C with CO(2) and read weekly. The results suggest that filtration of water for the isolation of mycobacteria is a more sensitive method for concentration than centrifugation. The addition of sodium thiosulfate may not be necessary and may reduce the yield. Middlebrook M7H10 and 7H11 were equally sensitive culture media. CPC decontamination, while effective for reducing growth of contaminants, also significantly reduces mycobacterial numbers. There was no difference at 3 weeks between the different incubation temperatures.
Stability study of azlocillin sodium in glass bottles and PVC bags containing intravenous admixtures.[Pubmed:7576447]
Boll Chim Farm. 1995 Sep;134(8):467-71.
The kinetics of degradation of azlocillin sodium in four intravenous admixtures was investigated at different temperatures. The effect of temperature has been determined and from this data, by applying of Arrhenius-law, the stability of azlocillin sodium at 25 degrees C has been predicted and the t90 was determined. Admixtures containing azlocillin sodium (0.01 g ml-1) were prepared in 0.9% sodium chloride injection, in 5% dextrose solution, in 5% levulose solution and in Ringer's lactate solution. The admixtures were stored at 30 degrees, 40 degrees and 50 degrees C in either polyvinyl chloride bags and glass bottles. The change in the initial azlocillin sodium concentration was related to the type of intravenous solution. No dependence with material of container was found. After 24 hours, the change in the initial concentration of penicillin was less than 10% of the initial concentration in 0.9% sodium chloride and 5% levulose solution. However in Ringer's lactate and 5% glucose solution the t90 was lower. These results were found in agreement with experimental ones obtained at room temperature.
Use of ciprofloxacin in cystic fibrosis patients.[Pubmed:2589355]
Am J Med. 1989 Nov 30;87(5A):123S-127S.
In order to evaluate the clinical efficacy and safety of oral ciprofloxacin in the treatment of acute pulmonary exacerbations of cystic fibrosis and trace the possible development of resistance over time, three trials were conducted. In an open-label, uncontrolled trial, 25 courses of ciprofloxacin were administered to 16 patients. Efficacy and safety were assessed based on changes in short-term clinical scores, white blood cell counts, Pseudomonas aeruginosa counts in sputum, pulmonary function tests, and standard serum chemistries and urinalysis that were performed before therapy, weekly during therapy, at the end of therapy, and at a seven-day follow-up visit after therapy. In an open-label, randomized, controlled study, the efficacy and tolerance of oral ciprofloxacin were compared with those of intravenous tobramycin and azlocillin. In another study, the rate of susceptibility of P. aeruginosa isolated from cystic fibrosis patients during more than two years of clinical use was determined. In the uncontrolled trial, ciprofloxacin therapy was associated with clinical improvement in most cases with changes in short-term clinical score and forced expiratory volume in one second being statistically significant (p less than 0.05). Twenty-five patients were entered in the controlled trial with 12 patients in each treatment group being evaluable. The groups were comparable based on admitting demographic and disease characteristics, and no differences in therapeutic response or side effects were noted between the two treatments (p greater than 0.5). Bacterial susceptibility to ciprofloxacin has remained relatively stable over time. Based on these results as well as those from similar evaluations, ciprofloxacin appears to be efficacious in the treatment of acute pulmonary exacerbations in adults with cystic fibrosis, producing responses similar to those observed with standard intravenous antibiotic therapy.
The use of aztreonam in the cystic fibrosis patient.[Pubmed:2682510]
Pediatr Infect Dis J. 1989 Sep;8(9 Suppl):S117-9; discussion S128-32.
Eradication of pulmonary infection by Pseudomonas aeruginosa in cystic fibrosis (CF) patients has long presented a significant challenge to the medical community. Many antimicrobial agents have proved incompletely effective against this persistent pathogen, and even the aminoglycosides, which represent the traditional therapy for such infections, have been associated with considerable toxicity and resistance. The monobactam antibacterial agent aztreonam is used both as single-agent therapy and in combination with other drugs. Several controlled, clinical trials have demonstrated the efficacy of aztreonam in the treatment of CF patients with pulmonary exacerbations caused by P. aeruginosa. The only side effect of aztreonam therapy commonly encountered in these studies was elevation of hepatic transaminase concentrations; this effect was of uncertain significance. It was concluded that aztreonam may offer clinical efficacy comparable to that provided by the combination of tobramycin plus azlocillin. Further, there does not seem to be any appreciable difference in the development of resistance to aztreonam compared with traditional therapies.


