DLPCLRH-1 agonist ligand CAS# 18194-25-7 |
- 7-Chlorokynurenic acid sodium salt
Catalog No.:BCC7757
CAS No.:1263094-00-3
- TFB-TBOA
Catalog No.:BCC5919
CAS No.:480439-73-4
- Dihydrokainic acid
Catalog No.:BCC6556
CAS No.:52497-36-6
- L-(-)-threo-3-Hydroxyaspartic acid
Catalog No.:BCC6565
CAS No.:7298-99-9
- WAY 213613
Catalog No.:BCC7442
CAS No.:868359-05-1
- LDN 212320
Catalog No.:BCC6361
CAS No.:894002-50-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 18194-25-7 | SDF | Download SDF |
| PubChem ID | 87506 | Appearance | Powder |
| Formula | C32H64NO8P | M.Wt | 621.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in ethanol | ||
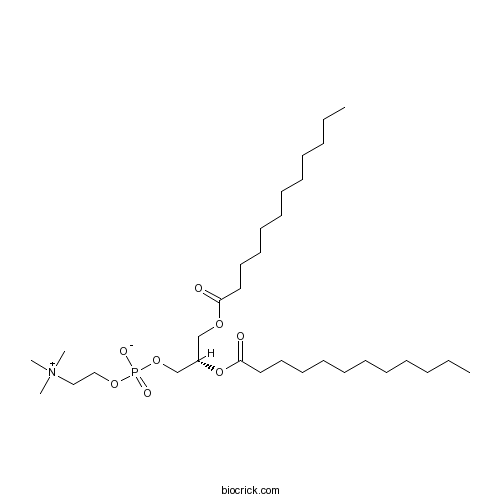
| Chemical Name | [(2S)-2,3-di(dodecanoyloxy)propyl] 2-(trimethylazaniumyl)ethyl phosphate | ||
| SMILES | CCCCCCCCCCCC(=O)OCC(COP(=O)([O-])OCC[N+](C)(C)C)OC(=O)CCCCCCCCCCC | ||
| Standard InChIKey | IJFVSSZAOYLHEE-PMERELPUSA-N | ||
| Standard InChI | InChI=1S/C32H64NO8P/c1-6-8-10-12-14-16-18-20-22-24-31(34)38-28-30(29-40-42(36,37)39-27-26-33(3,4)5)41-32(35)25-23-21-19-17-15-13-11-9-7-2/h30H,6-29H2,1-5H3/t30-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective agonist of the orphan nuclear receptor LRH-1 (liver receptor homolog-1, NR5A2) in vitro. Induces bile acid biosynthetic enzymes; increases bile acid and decreases hepatic triglycerides and serum glucose. Exhibits antidiabetic effects. |

DLPC Dilution Calculator

DLPC Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6082 mL | 8.0408 mL | 16.0816 mL | 32.1631 mL | 40.2039 mL |
| 5 mM | 0.3216 mL | 1.6082 mL | 3.2163 mL | 6.4326 mL | 8.0408 mL |
| 10 mM | 0.1608 mL | 0.8041 mL | 1.6082 mL | 3.2163 mL | 4.0204 mL |
| 50 mM | 0.0322 mL | 0.1608 mL | 0.3216 mL | 0.6433 mL | 0.8041 mL |
| 100 mM | 0.0161 mL | 0.0804 mL | 0.1608 mL | 0.3216 mL | 0.402 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: N/A
DLPC is an LRH-1 agonist ligand.
The orphan nuclear receptor liver receptor homolog-1 (LRH-1) is recognized as a critical regulator of bile salt biosynthesis. Bile salts are recognized increasingly as modulators of glucose and lipid metabolism in both human and animals.
In vitro: In a cell-free system, DLPC specifically bound to a recombinant LRH-1 ligand-binding domain. At functional level, DLPC was found to be a strong activator of both human and mouse LRH-1, while other nuclear receptors including FXR, CAR, PXR, PPARa and PPARc were all unaffected by DLPC in cell culture. In addition, DLPC induced the transactivation of the native mouse Shp and Oct4 promoters, consistent with previous studies on Lrh-1. In HepG2 cell line, DLPC could induce the expression of CYP8B1 [1].
In vivo: In mouse in vivo test, DLPC induced bile acid biosynthetic enzymes in mouse liver, lowered hepatic triglycerides and serum glucose, and increased bile acid levels. Moreover, DLPC treatment was also able to decrease hepatic steatosis and improve glucose homeostasis in two mouse models of insulin resistance [1].
Clinical trial: In a previous clinical study, single-dose inhalations of liposome preparations of Bec-DLPC and DLPC alone were administered. Results showed that no adverse clinical or laboratory events were observed. Thus, it was concluded that DLPC liposome aerosol was well tolerated in doses equivalent to those administered by MDIs for treatment of asthma [2].
References:
[1] Hohenester S,Beuers U. Phosphatidylcholines as regulators of glucose and lipid homeostasis: promises and potential risks. Hepatology.2011 Dec;54(6):2265-7.
[2] Waldrep JC,Gilbert BE,Knight CM,Black MB,Scherer PW,Knight V,Eschenbacher W. Pulmonary delivery of beclomethasone liposome aerosol in volunteers. Tolerance and safety. Chest.1997 Feb;111(2):316-23.
- KC 12291 hydrochloride
Catalog No.:BCC7618
CAS No.:181936-98-1
- Crotonoside
Catalog No.:BCN6281
CAS No.:1818-71-9
- 25-Anhydrocimigenol 3-O-beta-D-xyloside
Catalog No.:BCN3436
CAS No.:181765-11-7
- (-)-beta-Pinene
Catalog No.:BCN3857
CAS No.:18172-67-3
- Cyclopiazonic acid
Catalog No.:BCC6981
CAS No.:18172-33-3
- Interiotherins A
Catalog No.:BCN3093
CAS No.:181701-06-4
- Valdecoxib
Catalog No.:BCC4441
CAS No.:181695-72-7
- SB 228357
Catalog No.:BCC7036
CAS No.:181629-93-6
- tert-Butyldimethylsilyl Chloride
Catalog No.:BCC2796
CAS No.:18162-48-6
- Isonemerosin
Catalog No.:BCN6550
CAS No.:181524-79-8
- PD 160170
Catalog No.:BCC7284
CAS No.:181468-88-2
- Colutehydroquinone
Catalog No.:BCN8239
CAS No.:181311-16-0
- Sequirin C
Catalog No.:BCN4688
CAS No.:18194-29-1
- Fmoc-Dap-OH
Catalog No.:BCC3187
CAS No.:181954-34-7
- Naringenin-4',7-diacetate
Catalog No.:BCN1144
CAS No.:18196-13-9
- Angenomalin
Catalog No.:BCN8246
CAS No.:18199-64-9
- Ethyl beta-D-fructofuranoside
Catalog No.:BCN1145
CAS No.:1820-84-4
- BL 1249
Catalog No.:BCC7777
CAS No.:18200-13-0
- Meliasendanin D
Catalog No.:BCN7610
CAS No.:1820034-05-6
- KB-R7943 mesylate
Catalog No.:BCC1676
CAS No.:182004-65-5
- 6-Angeloyloxyditropan-3-yl itaconate
Catalog No.:BCN1867
CAS No.:182015-05-0
- Nitrosostromelin
Catalog No.:BCN1745
CAS No.:182064-61-5
- Quinovic acid 3-O-(3',4'-O-isopropylidene)-beta-D-fucopyranoside
Catalog No.:BCN1519
CAS No.:182132-59-8
- Nyssoside
Catalog No.:BCN1146
CAS No.:182138-70-1
Phase coexistence in films composed of DLPC and DPPC: a comparison between different model membrane systems.[Pubmed:24582710]
Biochim Biophys Acta. 2014 Jul;1838(7):1823-31.
For the biophysical study of membranes, a variety of model systems have been used to measure the different parameters and to extract general principles concerning processes that may occur in cellular membranes. However, there are very few reports in which the results obtained with the different models have been compared. In this investigation, we quantitatively compared the phase coexistence in Langmuir monolayers, freestanding bilayers and supported films composed of a lipid mixture of DLPC and DPPC. Two-phase segregation was observed in most of the systems for a wide range of lipid proportions using fluorescence microscopy. The lipid composition of the coexisting phases was determined and the distribution coefficient of the fluorescent probe in each phase was quantified, in order to explore their thermodynamic properties. The comparison between systems was carried out at 30mN/m, since it is accepted that at this or higher lateral pressures, the mean molecular area in bilayers is equivalent to that observed in monolayers. Our study showed that while Langmuir monolayers and giant unilamellar vesicles had a similar phase behavior, supported films showed a different composition of the phases with the distribution coefficient of the fluorescent probe being close to unity. Our results suggest that, in supported membranes, the presence of the rigid substrate may have led to a stiffening of the liquid-expanded phase due to a loss in the degrees of freedom of the lipids as a consequence of the proximity of the solid material.
Molecular dynamics investigations of PRODAN in a DLPC bilayer.[Pubmed:22329741]
J Phys Chem B. 2012 Mar 8;116(9):2713-21.
Molecular dynamics computer simulations have been performed to identify preferred positions of the fluorescent probe PRODAN in a fully hydrated DLPC bilayer in the fluid phase. In addition to the intramolecular charge-transfer first vertical excited state, we considered different charge distributions for the electronic ground state of the PRODAN molecule by distinct atomic charge models corresponding to the probe molecule in vacuum as well as polarized in a weak and a strong dielectric solvent (cyclohexane and water). Independent on the charge distribution model of PRODAN, we observed a preferential orientation of this molecule in the bilayer with the dimethylamino group pointing toward the membrane's center and the carbonyl oxygen toward the membrane's interface. However, changing the charge distribution model of PRODAN, independent of its initial position in the equilibrated DLPC membrane, we observed different preferential positions. For the ground state representation without polarization and the in-cyclohexane polarization, the probe maintains its position close to the membrane's center. Considering the in-water polarization model, the probe approaches more of the polar headgroup region of the bilayer, with a strong structural correlation with the choline group, exposing its oxygen atom to water molecules. PRODAN's representation of the first vertical excited state with the in-water polarization also approaches the polar region of the membrane with the oxygen atom exposed to the bilayer's hydration shell. However, this model presents a stronger structural correlation with the phosphate groups than the ground state. Therefore, we conclude that the orientation of the PRODAN molecule inside the DLPC membrane is well-defined, but its position is very sensitive to the effect of the medium polarization included here by different models for the atomic charge distribution of the probe.
Effect of products of PLA2 catalyzed hydrolysis of DLPC on motion of rising bubbles.[Pubmed:25724770]
Colloids Surf B Biointerfaces. 2015 Apr 1;128:261-267.
Local velocities of rising bubbles decrease with the increasing concentration in solution of surface-active, water-soluble species. Therefore, it is possible to use this phenomenon to monitor products of enzymatic reactions, which meet such criteria. In this study, hydrolysis of 1,2-dilauroyl-sn-glycero-3-phosphatidylcholine (DLPC) catalyzed by calcium-dependent phospholipase A2 (PLA2) (EC3.1.1.4) from porcine pancreas was used as model reaction. The products of this reaction are lauric acid (LA) and 1-lauroyl-2-hydroxy-sn-glycero-3-phosphatidylcholine (Lyso-PC). DLPC was dispersed in a chloroform/methanol mixture that was spread on a free PLA2 solution surface. Air bubbles were then formed at a capillary orifice and the local velocity of rising bubbles as a function of the distance from the capillary tip was monitored. Local velocity profiles were compared with profiles recorded for solutions of pure enzymatic reaction products and their mixtures. Our experiments showed that the product, which had a dominating effect on bubble motion retardation, was lyso-phosphatidylcholine. This can be explained by differences in the kinetics of lauric acid and lyso-phosphatidylcholine transfer from the spread layer to the solution.
Evidence for the formation of symmetric and asymmetric DLPC-DAPC lipid bilayer domains.[Pubmed:23867833]
Cell Physiol Biochem. 2013;32(1):46-52.
BACKGROUND/AIMS: We investigated if mixtures of the phosphatidylcholine (PC) lipids 1,2-dilauroyl-sn-glycero-3-phosphocholine (C12:0 PC; DLPC) and 1,2-diarachidoyl-sn-glycero-3-phosphocholine (C20:0 PC; DAPC), which differ by eight methylene groups in acyl chain length, lead to the spontaneous formation of distinct lipid rafts and asymmetric bilayers. METHODS: The experiments were performed using Atomic Force Microscopy (AFM). RESULTS: We show that DLPC and DAPC mixed at a molar ratio of 1:1 lead to the formation of single, double and triple bilayers with peaks at 6.14 +/- 0.11, 13.27 +/- 0.17 and 20.54 +/- 0.46 nm, respectively (n=750). Within these formations discrete height steps of 0.92 nm can be resolved (n=422). CONCLUSION: The most frequently observed height steps value of 0.92 nm matches best with the calculated mean lipid hydrophobic thickness difference for asymmetric C12:0 PC and C20:0 PC lipid bilayers of 0.88 nm. This indicates the ability of DLPC and DAPC to form asymmetric lipid bilayers.
A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects.[Pubmed:21614002]
Nature. 2011 May 25;474(7352):506-10.
Nuclear hormone receptors regulate diverse metabolic pathways and the orphan nuclear receptor LRH-1 (also known as NR5A2) regulates bile acid biosynthesis. Structural studies have identified phospholipids as potential LRH-1 ligands, but their functional relevance is unclear. Here we show that an unusual phosphatidylcholine species with two saturated 12 carbon fatty acid acyl side chains (dilauroyl phosphatidylcholine (DLPC)) is an LRH-1 agonist ligand in vitro. DLPC treatment induces bile acid biosynthetic enzymes in mouse liver, increases bile acid levels, and lowers hepatic triglycerides and serum glucose. DLPC treatment also decreases hepatic steatosis and improves glucose homeostasis in two mouse models of insulin resistance. Both the antidiabetic and lipotropic effects are lost in liver-specific Lrh-1 knockouts. These findings identify an LRH-1 dependent phosphatidylcholine signalling pathway that regulates bile acid metabolism and glucose homeostasis.


