Decanedioic acidCAS# 111-20-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111-20-6 | SDF | Download SDF |
| PubChem ID | 5192 | Appearance | Powder |
| Formula | C10H18O4 | M.Wt | 202.3 |
| Type of Compound | Lipids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | decanedioic acid | ||
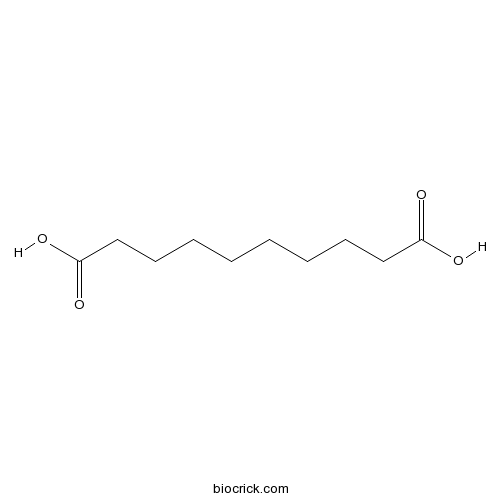
| SMILES | C(CCCCC(=O)O)CCCC(=O)O | ||
| Standard InChIKey | CXMXRPHRNRROMY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C10H18O4/c11-9(12)7-5-3-1-2-4-6-8-10(13)14/h1-8H2,(H,11,12)(H,13,14) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Decanedioic acid is a saturated, straight-chain naturally occurring dicarboxylic acid, it exhibits significantly higher anti-HIV activity. |
| Targets | HIV |
| In vitro | Synthesis and Biological Evaluation of 5'-O-Dicarboxylic Fatty Acyl Monoester Derivatives of Anti-HIV Nucleoside Reverse Transcriptase Inhibitors.[Pubmed: 24791029]Tetrahedron Lett. 2014 Mar 19;55(12):1983-1986.A number of 5'-O-dicarboxylic fatty acyl monoester derivatives of 3'-azido-3'-deoxythymidine (zidovudine, AZT), 2',3'-didehydro-2',3'-dideoxythymidine (stavudine, d4T), and 3'-fluoro-3'-deoxythymidine (alovudine, FLT) were synthesized to improve the lipophilicity and potentially the cellular delivery of parent polar 2', 3'-dideoxynucleoside (ddN) analogues. |
| Structure Identification | AAPS PharmSciTech. 2014 Feb;15(1):111-20.Identification of unknown impurity of azelaic acid in liposomal formulation assessed by HPLC-ELSD, GC-FID, and GC-MS.[Pubmed: 24166667]The identification of new contaminants is critical in the development of new medicinal products. Many impurities, such as pentanedioic acid, hexanedioic acid, heptanedioic acid, octanedioic acid, Decanedioic acid, unDecanedioic acid, doDecanedioic acid, triDecanedioic acid, and tetraDecanedioic acid, have been identified in samples of azelaic acid. |

Decanedioic acid Dilution Calculator

Decanedioic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.9432 mL | 24.7158 mL | 49.4315 mL | 98.8631 mL | 123.5788 mL |
| 5 mM | 0.9886 mL | 4.9432 mL | 9.8863 mL | 19.7726 mL | 24.7158 mL |
| 10 mM | 0.4943 mL | 2.4716 mL | 4.9432 mL | 9.8863 mL | 12.3579 mL |
| 50 mM | 0.0989 mL | 0.4943 mL | 0.9886 mL | 1.9773 mL | 2.4716 mL |
| 100 mM | 0.0494 mL | 0.2472 mL | 0.4943 mL | 0.9886 mL | 1.2358 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Squalene
Catalog No.:BCN5995
CAS No.:111-02-4
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tunicamycin
Catalog No.:BCC7699
CAS No.:11089-65-9
- [Sar9,Met(O2)11]-Substance P
Catalog No.:BCC6960
CAS No.:110880-55-2
- Wilforine
Catalog No.:BCN5994
CAS No.:11088-09-8
- Entrectinib
Catalog No.:BCC6410
CAS No.:1108743-60-7
- Sparfloxacin
Catalog No.:BCC4848
CAS No.:110871-86-8
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
- Cinobufotalin
Catalog No.:BCN2283
CAS No.:1108-68-5
- Garcinexanthone A
Catalog No.:BCN5993
CAS No.:1107620-67-6
- Asebotin
Catalog No.:BCN7233
CAS No.:11075-15-3
- beta-Escin
Catalog No.:BCC8172
CAS No.:11072-93-8
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
- Pioglitazone
Catalog No.:BCC4927
CAS No.:111025-46-8
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Ruthenium Red
Catalog No.:BCC7067
CAS No.:11103-72-3
Identification of unknown impurity of azelaic acid in liposomal formulation assessed by HPLC-ELSD, GC-FID, and GC-MS.[Pubmed:24166667]
AAPS PharmSciTech. 2014 Feb;15(1):111-20.
The identification of new contaminants is critical in the development of new medicinal products. Many impurities, such as pentanedioic acid, hexanedioic acid, heptanedioic acid, octanedioic acid, Decanedioic acid, unDecanedioic acid, doDecanedioic acid, triDecanedioic acid, and tetraDecanedioic acid, have been identified in samples of azelaic acid. The aim of this study was to identify impurities observed during the stability tests of a new liposomal dosage form of azelaic acid that is composed of phosphatidylcholine and a mixture of ethyl alcohol and water, using high-performance liquid chromatography with evaporative light-scattering detector (HPLC-ELSD), gas chromatography-flame ionisation detection (GC-FID), and gas chromatography-mass spectrometry (GC-MS) methods. During the research and development of a new liposomal formulation of azelaic acid, we developed a method for determining the contamination of azelaic acid using HPLC-ELSD. During our analytical tests, we identified a previously unknown impurity of a liposomal preparation of azelaic acid that appeared in the liposomal formulation of azelaic acid during preliminary stability studies. The procedure led to the conclusion that the impurity was caused by the reaction of azelaic acid with one of the excipients that was applied in the product. The impurity was finally identified as an ethyl monoester of azelaic acid. The identification procedure of this compound was carried out in a series of experiments comparing the chromatograms that were obtained via the following chromatographic methods: HPLC-ELSD, GC-FID, and GC-MS. The final identification of the compound was carried out by GC with MS.
Synthesis and Biological Evaluation of 5'-O-Dicarboxylic Fatty Acyl Monoester Derivatives of Anti-HIV Nucleoside Reverse Transcriptase Inhibitors.[Pubmed:24791029]
Tetrahedron Lett. 2014 Mar 19;55(12):1983-1986.
A number of 5'-O-dicarboxylic fatty acyl monoester derivatives of 3'-azido-3'-deoxythymidine (zidovudine, AZT), 2',3'-didehydro-2',3'-dideoxythymidine (stavudine, d4T), and 3'-fluoro-3'-deoxythymidine (alovudine, FLT) were synthesized to improve the lipophilicity and potentially the cellular delivery of parent polar 2', 3'-dideoxynucleoside (ddN) analogues. The compounds were evaluated for their anti-HIV activity. Three different fatty acids with varying chain length of suberic acid (octanedioic acid), sebacic acid (Decanedioic acid), and doDecanedioic acid were used for the conjugation with the nucleosides. The compounds were evaluated for anti-HIV activity and cytotoxicity. All dicarboxylic ester conjugates of nucleosides exhibited significantly higher anti-HIV activity than that of the corresponding parent nucleoside analogs. Among all the tested conjugates, 5'-O-suberate derivative of AZT (EC50 = 0.10 nM) was found to be the most potent compound and showed 80-fold higher anti-HIV activity than AZT without any significant toxicity (TC50 > 500 nM).


