PioglitazoneCAS# 111025-46-8 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111025-46-8 | SDF | Download SDF |
| PubChem ID | 4829 | Appearance | Powder |
| Formula | C19H20N2O3S | M.Wt | 356.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | U 72107 | ||
| Solubility | DMSO : 62.5 mg/mL (175.35 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
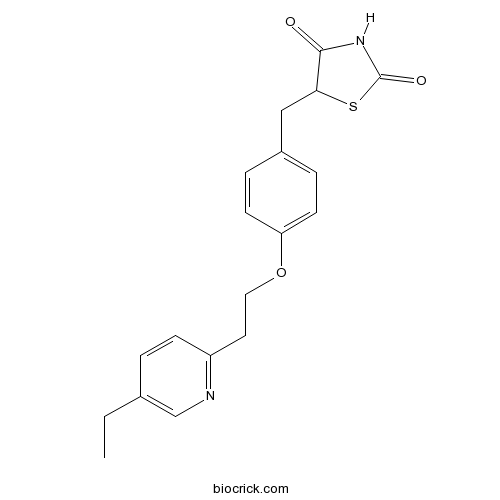
| Chemical Name | 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione | ||
| SMILES | CCC1=CN=C(C=C1)CCOC2=CC=C(C=C2)CC3C(=O)NC(=O)S3 | ||
| Standard InChIKey | HYAFETHFCAUJAY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C19H20N2O3S/c1-2-13-3-6-15(20-12-13)9-10-24-16-7-4-14(5-8-16)11-17-18(22)21-19(23)25-17/h3-8,12,17H,2,9-11H2,1H3,(H,21,22,23) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pioglitazone is a potent and selective PPARγ agonist with high affinity binding to the PPARγ ligand-binding domain with EC50 of 0.93 and 0.99 μM for human and mouse PPARγ, respectively.In Vitro:AGEs-induced beta cell necrosis is completely abrogated by adding Pioglitazone to the AGEs culture medium. Furthermore Pioglitazone completely prevented any AGEs-induced increment in caspase-3 activation, thereby restoring caspase-3 activity to the same levels as the control cells. As expected AG is able to counteract AGEs-induced impaired viability[2].In Vivo:The serum-free fatty acid and triglyceride levels as well as adipocyte sizes in ob/ob and adipo-/- ob/ob mice are unchanged after 10 mg/kg Pioglitazone but are significantly reduced to a similar degree after 30 mg/kg Pioglitazone. Moreover, the expressions of TNFα and resistin in adipose tissues of ob/ob and adipo-/- ob/ob mice are unchanged after 10 mg/kg Pioglitazone but are decreased after 30 mg/kg Pioglitazone. Thus, Pioglitazone-induced amelioration of insulin resistance and diabetes may occur adiponectin dependently in the liver and adiponectin independently in skeletal muscle[3]. Pioglitazone (10 mg/kg per d) treatment significantly attenuates the loss of body weight (BW) and cardiac hypertrophy. Pioglitazone treatment significantly reduces the elevated serum glucose levels and markedly improved the associated dyslipidemia. Furthermore, there is a slight but significant increase in serum creatinine level in D rats over their N controls (P <0.05). However, a marked renal dysfunction is observed in diabetic nephropathic (DN) group (P<0.05). Moreover, DN rats exhibits the highest serum activity of CK-MB, relative to both N and D rats (P<0.05). Pioglitazone is able to decrease the elevated serum levels of both creatinine and creatine kinase-MB (CK-MB)[4]. References: | |||||

Pioglitazone Dilution Calculator

Pioglitazone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8055 mL | 14.0276 mL | 28.0552 mL | 56.1104 mL | 70.138 mL |
| 5 mM | 0.5611 mL | 2.8055 mL | 5.611 mL | 11.2221 mL | 14.0276 mL |
| 10 mM | 0.2806 mL | 1.4028 mL | 2.8055 mL | 5.611 mL | 7.0138 mL |
| 50 mM | 0.0561 mL | 0.2806 mL | 0.5611 mL | 1.1222 mL | 1.4028 mL |
| 100 mM | 0.0281 mL | 0.1403 mL | 0.2806 mL | 0.5611 mL | 0.7014 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pioglitazone (U 72107) is a selective peroxisome proliferator-activated receptor gamma(PPARγ) stimulator.
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- Decanedioic acid
Catalog No.:BCN5996
CAS No.:111-20-6
- Squalene
Catalog No.:BCN5995
CAS No.:111-02-4
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Ruthenium Red
Catalog No.:BCC7067
CAS No.:11103-72-3
- Annonacin
Catalog No.:BCN4734
CAS No.:111035-65-5
- 2-(2'-Hydroxy-4'-methylphenyl)propionic acid
Catalog No.:BCN7980
CAS No.:111044-84-9
- Ginkgolic acid C17:1
Catalog No.:BCN5334
CAS No.:111047-30-4
- N-Benzoyl-2-hydroxy-2-phenylethylamine
Catalog No.:BCN1622
CAS No.:111059-46-2
- Fmoc-D-Lys(Trt)-OH
Catalog No.:BCC2594
CAS No.:111061-54-2
- Fmoc-Ser(Trt)-OH
Catalog No.:BCC3546
CAS No.:111061-56-4
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- FERb 033
Catalog No.:BCC7701
CAS No.:1111084-78-6
- 14-Hydroxy sprengerinin C
Catalog No.:BCN2777
CAS No.:1111088-89-1
- NF 110
Catalog No.:BCC7404
CAS No.:111150-22-2
Pioglitazone utilization, efficacy & safety in Indian type 2 diabetic patients: A systematic review & comparison with European Medicines Agency Assessment Report.[Pubmed:28361819]
Indian J Med Res. 2016 Nov;144(5):672-681.
BACKGROUND & OBJECTIVES: With Pioglitazone ban and subsequent revoking in India along with varying regulatory decisions in other countries, it was decided to carry out a systematic review on its safety, efficacy and drug utilization in patients with type 2 diabetes mellitus (T2DM) in India and compare with the data from the European Medicines Agency Assessment Report (EMA-AR). METHODS: Systematic review was performed as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, searching Medline/PubMed, Google Scholar and Science Direct databases using 'Pioglitazone AND India AND human' and 'Pioglitazone AND India AND human AND patient' and compared with EMA-AR. Spontaneous reports in World Health Organization VigiBase from India were compared with VigiBase data from other countries. RESULTS: Sixty six publications, 26 (efficacy), 32 (drug utilization) and eight (safety), were retrieved. In India, Pioglitazone was used at 15-30 mg/day mostly with metformin and sulphonylurea, being prescribed to 26.7 and 8.4 per cent patients in north and south, respectively. The efficacy in clinical trials (CTs) was similar to those in EMA-AR. Incidence of bladder cancer in Pioglitazone exposed and non-exposed patients was not significantly different in an Indian retrospective cohort study. There were two cases and a series of eight cases of bladder cancer published but none reported in VigiBase. INTERPRETATION & CONCLUSIONS: In India, probably due to lower dose, lower background incidence of bladder cancer and smaller sample size in epidemiological studies, association of bladder cancer with Pioglitazone was not found to be significant. Reporting of CTs and adverse drug reactions to Clinical Trials Registry of India and Pharmacovigilance Programme of India, respectively, along with compliance studies with warning given in package insert and epidemiological studies with larger sample size are needed.
Pioglitazone treatment following spinal cord injury maintains acute mitochondrial integrity and increases chronic tissue sparing and functional recovery.[Pubmed:28365473]
Exp Neurol. 2017 Jul;293:74-82.
Pioglitazone is an FDA-approved PPAR-gamma agonist drug used to treat diabetes, and it has demonstrated neuroprotective effects in multiple models of central nervous system (CNS) injury. Acute treatment after spinal cord injury (SCI) in rats is reported to suppress neuroinflammation, rescue injured tissues, and improve locomotor recovery. In the current study, we additionally assessed the protective efficacy of Pioglitazone treatment on acute mitochondrial respiration, as well as functional and anatomical recovery after contusion SCI in adult male C57BL/6 mice. Mice received either vehicle or Pioglitazone (10mg/kg) at either 15min or 3h after injury (75kdyn at T9) followed by a booster at 24h post-injury. At 25h, mitochondria were isolated from spinal cord segments centered on the injury epicenters and assessed for their respiratory capacity. Results showed significantly compromised mitochondrial respiration 25h following SCI, but Pioglitazone treatment that was initiated either at 15min or 3h post-injury significantly maintained mitochondrial respiration rates near sham levels. A second cohort of injured mice received Pioglitazone at 15min post injury, then once a day for 5days post-injury to assess locomotor recovery and tissue sparing over 4weeks. Compared to vehicle, Pioglitazone treatment resulted in significantly greater recovery of hind-limb function over time, as determined by serial locomotor BMS assessments and both terminal BMS subscores and gridwalk performance. Such improvements correlated with significantly increased grey and white matter tissue sparing, although Pioglitazone treatment did not abrogate long-term injury-induced inflammatory microglia/macrophage responses. In sum, Pioglitazone significantly increased functional neuroprotection that was associated with remarkable maintenance of acute mitochondrial bioenergetics after traumatic SCI. This sets the stage for dose-response and delayed administration studies to maximize Pioglitazone's efficacy for SCI while elucidating the precise role that mitochondria play in governing its neuroprotection; the ultimate goal to develop novel therapeutics that specifically target mitochondrial dysfunction.


