Ruthenium RedBlocks capsaicin-activated cation channels CAS# 11103-72-3 |
- BMN-673 8R,9S
Catalog No.:BCC1422
CAS No.:1207456-00-5
- ME0328
Catalog No.:BCC3995
CAS No.:1445251-22-8
- Rucaparib (free base)
Catalog No.:BCC4012
CAS No.:283173-50-2
- PJ34 hydrochloride
Catalog No.:BCC2210
CAS No.:344458-15-7
- Olaparib (AZD2281, Ku-0059436)
Catalog No.:BCC2206
CAS No.:763113-22-0
- NU 1025
Catalog No.:BCC2454
CAS No.:90417-38-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

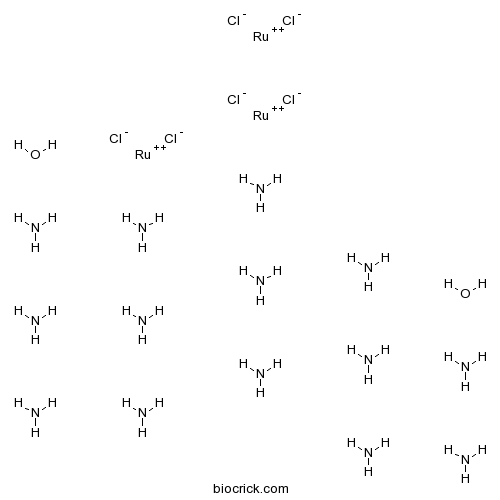
| Cas No. | 11103-72-3 | SDF | Download SDF |
| PubChem ID | 16218584 | Appearance | Powder |
| Formula | H42N14O2Ru3Cl6 | M.Wt | 786.35 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water | ||
| Chemical Name | azane;ruthenium(2+);hexachloride;dihydrate | ||
| SMILES | N.N.N.N.N.N.N.N.N.N.N.N.N.N.O.O.[Cl-].[Cl-].[Cl-].[Cl-].[Cl-].[Cl-].[Ru+2].[Ru+2].[Ru+2] | ||
| Standard InChIKey | JQJSTVUROJELSR-UHFFFAOYSA-H | ||
| Standard InChI | InChI=1S/6ClH.14H3N.2H2O.3Ru/h6*1H;14*1H3;2*1H2;;;/q;;;;;;;;;;;;;;;;;;;;;;3*+2/p-6 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Blocks Ca2+ uptake and release from mitochondria, and Ca2+ release from ryanodine-sensitive intracellular stores. Also blocks cell membrane-located capsaicin-activated cation channels (IC50 = 14 nM) and voltage-sensitive Ca2+ channels to inhibit neurotransmitter release. |

Ruthenium Red Dilution Calculator

Ruthenium Red Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.2717 mL | 6.3585 mL | 12.717 mL | 25.434 mL | 31.7925 mL |
| 5 mM | 0.2543 mL | 1.2717 mL | 2.5434 mL | 5.0868 mL | 6.3585 mL |
| 10 mM | 0.1272 mL | 0.6358 mL | 1.2717 mL | 2.5434 mL | 3.1792 mL |
| 50 mM | 0.0254 mL | 0.1272 mL | 0.2543 mL | 0.5087 mL | 0.6358 mL |
| 100 mM | 0.0127 mL | 0.0636 mL | 0.1272 mL | 0.2543 mL | 0.3179 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Pioglitazone
Catalog No.:BCC4927
CAS No.:111025-46-8
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- Decanedioic acid
Catalog No.:BCN5996
CAS No.:111-20-6
- Annonacin
Catalog No.:BCN4734
CAS No.:111035-65-5
- 2-(2'-Hydroxy-4'-methylphenyl)propionic acid
Catalog No.:BCN7980
CAS No.:111044-84-9
- Ginkgolic acid C17:1
Catalog No.:BCN5334
CAS No.:111047-30-4
- N-Benzoyl-2-hydroxy-2-phenylethylamine
Catalog No.:BCN1622
CAS No.:111059-46-2
- Fmoc-D-Lys(Trt)-OH
Catalog No.:BCC2594
CAS No.:111061-54-2
- Fmoc-Ser(Trt)-OH
Catalog No.:BCC3546
CAS No.:111061-56-4
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- FERb 033
Catalog No.:BCC7701
CAS No.:1111084-78-6
- 14-Hydroxy sprengerinin C
Catalog No.:BCN2777
CAS No.:1111088-89-1
- NF 110
Catalog No.:BCC7404
CAS No.:111150-22-2
- PF-04880594
Catalog No.:BCC3998
CAS No.:1111636-35-1
- 1,2-O-Dilinoleoyl-3-O-beta-D-galactopyranosylracglycerol
Catalog No.:BCN6768
CAS No.:111187-15-6
Effect of ruthenium red, a ryanodine receptor antagonist in experimental diabetes induced vascular endothelial dysfunction and associated dementia in rats.[Pubmed:27262216]
Physiol Behav. 2016 Oct 1;164(Pt A):140-50.
Diabetes mellitus is considered as a main risk factor for vascular dementia. In the past, we have reported the induction of vascular dementia by experimental diabetes. This study investigates the efficacy of a Ruthenium Red, a ryanodine receptor antagonist and pioglitazone in the pharmacological interdiction of pancreatectomy diabetes (PaD) induced vascular endothelial dysfunction and subsequent vascular dementia in rats. Attentional set shifting and Morris water-maze test were used for assessment of learning and memory. Vascular endothelial function, blood brain barrier permeability, serum glucose, serum nitrite/nitrate, oxidative stress (viz. aortic superoxide anion, brain thiobarbituric acid reactive species and brain glutathione), brain calcium and inflammation (myeloperoxidase) were also estimated. PaD rats have shown impairment of endothelial function, blood brain barrier permeability, learning and memory along with an increase in brain inflammation, oxidative stress and calcium. Administration of Ruthenium Red and pioglitazone has significantly attenuated PaD induced impairment of learning, memory, blood brain barrier permeability, endothelial function and biochemical parameters. It may be concluded that Ruthenium Red, a ryanodine receptor antagonist and pioglitazone, a PPAR-gamma agonist may be considered as potent pharmacological agent for the management of PaD induced endothelial dysfunction and subsequent vascular dementia. Ryanodine receptor may be explored further for their possible benefits in vascular dementia.
Red-Emitting Ruthenium(II) and Iridium(III) Complexes as Phosphorescent Probes for Methylglyoxal in Vitro and in Vivo.[Pubmed:28098984]
Inorg Chem. 2017 Feb 6;56(3):1309-1318.
Transition-metal complexes, ruthenium(II) and iridium(III) complexes in particular, with fascinating triplet emissions are rapidly emerging as important phosphorescent dyes for application in the sensing and imaging of biological makers in live cells and organisms. In this contribution, two red-emitting transition-metal complexes, [Ru(bpy)2(DA-phen)](PF6)2 and [Ir(ppy)2(DA-phen)](PF6) (bpy = 2,2'-bipyridine, DA-phen = 4,5-diamino-1,10-phenanthroline, and ppy = 2-phenylpyridine), were designed and synthesized as phosphorescent probes for the highly sensitive and selective detection of methylglyoxal (MGO), an essential biomarker in the etiopathogenesis of several diseases. Both probes showed weak emissions in aqueous media because of the existence of an effective photoinduced-electron-transfer process, while their emissions could be remarkably enhanced upon the addition of MGO. The photophysical and electrochemical properties, as well as phosphorescent responses of the probes toward MGO, were examined. The ground- and excited-state properties of the probes and their reaction products with MGO, [Ru(bpy)2(MP-phen)](PF6)2 and [Ir(ppy)2(MP-phen)](PF6) (MP-phen = 2-methylpyrazino-1,10-phenanthroline), the sensing mechanism, and several important experimental facts were investigated and validated using density functional theory (DFT)/time-dependent DFT computations. The results indicated that the phosphorescence switch-ON is due to the elimination of electron transfer and followed the reestablishment of emissive triplet excited states. To evaluate the feasibility of [Ru(bpy)2(DA-phen)](PF6)2 and [Ir(ppy)2(DA-phen)](PF6) as bioprobes, their cytotoxicity was examined, and their applicability for visualizing intracellular and in vivo MGO was demonstrated.
Gadolinium and ruthenium red attenuate remote hind limb preconditioning-induced cardioprotection: possible role of TRP and especially TRPV channels.[Pubmed:27118661]
Naunyn Schmiedebergs Arch Pharmacol. 2016 Aug;389(8):887-96.
Remote ischemic preconditioning is a well reported therapeutic strategy that induces cardioprotective effects but the underlying intracellular mechanisms have not been widely explored. The current study was designed to investigate the involvement of TRP and especially TRPV channels in remote hind limb preconditioning-induced cardioprotection. Remote hind limb preconditioning stimulus (4 alternate cycles of inflation and deflation of 5 min each) was delivered using a blood pressure cuff tied on the hind limb of the anesthetized rat. Using Langendorff's system, the heart was perfused and subjected to 30-min ischemia and 120-min reperfusion. The myocardial injury was assessed by measuring infarct size, lactate dehydrogenase (LDH), creatine kinase (CK), LVDP, +dp/dtmax, -dp/dtmin, heart rate, and coronary flow rate. Gadolinium, TRP blocker, and Ruthenium Red, TRPV channel blocker, were employed as pharmacological tools. Remote hind limb preconditioning significantly reduced the infarct size, LDH release, CK release and improved coronary flow rate, hemodynamic parameters including LVDP, +dp/dtmax, -dp/dtmin, and heart rate. However, gadolinium (7.5 and 15 mg kg(-1)) and Ruthenium Red (4 and 8 mg kg(-1)) significantly attenuated the cardioprotective effects suggesting the involvement of TRP especially TRPV channels in mediating remote hind limb preconditioning-induced cardioprotection. Remote hind limb preconditioning stimulus possibly activates TRPV channels on the heart or sensory nerve fibers innervating the heart to induce cardioprotective effects. Alternatively, remote hind limb preconditioning stimulus may also activate the mechanosensitive TRP and especially TRPV channels on the sensory nerve fibers innervating the skeletal muscles to trigger cardioprotective neurogenic signaling cascade. The cardioprotective effects of remote hind limb preconditioning may be mediated via activation of mechanosensitive TRP and especially TRPV channels.
Ruthenium-tris(bipyridine) complexes with multiple redox-active amine substituents: tuning of spin density distribution and deep-red to NIR electrochromism and electrofluorochromism.[Pubmed:27240642]
Dalton Trans. 2016 Jun 21;45(25):10136-40.
In response to the application of low electrochemical potentials, ruthenium-tris(bipyridine) complexes decorated with multiple electron-rich and redox-active amine substituents show reversible absorption and emission spectral changes in the deep-red to NIR region. The number of amine substituents strongly affects the electrochemical and spectroscopic properties and the spin density distributions of the complex in the one-electron-oxidized state.
Ruthenium red modifies the cardiac and skeletal muscle Ca(2+) release channels (ryanodine receptors) by multiple mechanisms.[Pubmed:10551824]
J Biol Chem. 1999 Nov 12;274(46):32680-91.
The effects of Ruthenium Red (RR) on the skeletal and cardiac muscle ryanodine receptors (RyRs) were studied in vesicle-Ca(2+) flux, [(3)H]ryanodine binding, and single channel measurements. In vesicle-Ca(2+) flux measurements, RR was more effective in inhibiting RyRs at 0.2 microM than 20 microM free Ca(2+). [(3)H]Ryanodine binding measurements suggested noncompetitive interactions between RR inhibition and Ca(2+) regulatory sites of RyRs. In symmetric 0.25 M KCl with 10-20 microM cytosolic Ca(2+), cytosolic RR decreased single channel activities at positive and negative holding potentials. In close to fully activated skeletal (20 microM Ca(2+) + 2 mM ATP) and cardiac (200 microM Ca(2+)) RyRs, cytosolic RR induced a predominant subconductance at a positive but not negative holding potential. Lumenal RR induced a major subconductance in cardiac RyR at negative but not positive holding potentials and several subconductances in skeletal RyR. The RR-related subconductances of cardiac RyR showed a nonlinear voltage dependence, and more than one RR molecule appeared to be involved in their formation. Cytosolic and lumenal RR also induced subconductances in Ca(2+)-conducting skeletal and cardiac RyRs recorded at 0 mV holding potential. These results suggest that RR inhibits RyRs and induces subconductances by binding to cytosolic and lumenal sites of skeletal and cardiac RyRs.
Ruthenium red as a capsaicin antagonist.[Pubmed:1715010]
Life Sci. 1991;49(12):849-56.
Definition of the physiological and pharmacological properties of primary afferent neurons by the use of capsaicin and its analogues (e.g. resiniferatoxin) has represented one of the most active areas of research of the last decade (1-4 for reviews). In the past 3 years many important advancements have been made in this field, dealing with: a) discovery of the capsaicin (or 'vanilloid' receptor (5); b) discovery of capsazepine as a competitive receptor antagonist at the vanilloid receptor (6); c) definition of the cation channel coupled with the vanilloid receptor and the ionic basis for excitation and "desensitization" of primary afferents by capsaicin and related substances (7,8) and d) discovery of Ruthenium Red as a functional capsaicin antagonist. The aim of the present article is to briefly review the pharmacology of Ruthenium Red as a capsaicin antagonist and attempting to define the usefulness and the limits of this substance as a tool in sensory neuron research.
Pathway for uncoupler-induced calcium efflux in rat liver mitochondria: inhibition by ruthenium red.[Pubmed:6202317]
Biochemistry. 1984 Apr 10;23(8):1645-51.
The rate of uncoupler-induced Ca2+ efflux from rat liver mitochondria is increased by acetate and decreased by phosphate. This effect depends on a shift of the apparent Km, which is increased by phosphate and decreased by acetate, while the Vmax is not modified. The modification of the apparent Km by permeant anions presumably reflects changes in the concentration of matrix free Ca2+. A major part of uncoupler-induced Ca2+ efflux is sensitive to Ruthenium Red, the specific inhibitor of the Ca2+ uniporter , but an apparent insensitivity is observed when the H+ permeability is rate limiting in the process of Ca2+ efflux. The titer of uncoupler required for maximal stimulation of Ca2+ efflux increases with the Ca2+ load and may be 1-2 orders of magnitude higher than that required for maximal stimulation of respiration. On the other hand, when the uncoupler concentration is raised above 10(-6) M, the process of Ca2+ efflux becomes again Ruthenium Red insensitive. The Ruthenium Red inhibition of uncoupler-induced Ca2+ efflux is time dependent, in that the degree of inhibition exerted by low amounts of Ruthenium Red increases with the incubation time. Since the inhibition of the rate of Ca2+ influx undergoes a parallel relief, it is possible that Ruthenium Red moves from the cytosolic to the matrix side of the inner membrane. It is concluded that, in native mitochondria, uncoupler-induced Ca2+ efflux occurs via reversal of the uniport Ca2+ carrier, and not through activation of an independent pathway.


