DiethanolamineCAS# 111-42-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111-42-2 | SDF | Download SDF |
| PubChem ID | 8113 | Appearance | Oil |
| Formula | C4H11NO2 | M.Wt | 105.14 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
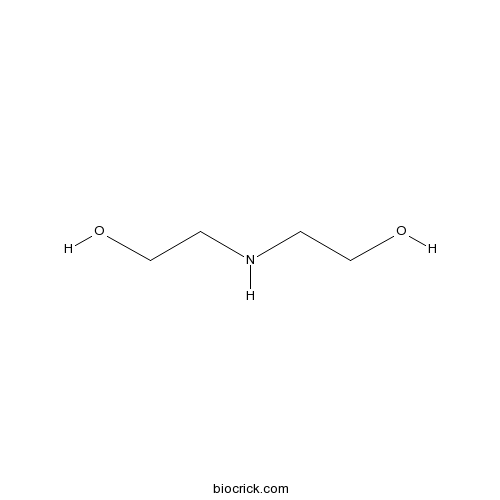
| Chemical Name | 2-(2-hydroxyethylamino)ethanol | ||
| SMILES | C(CO)NCCO | ||
| Standard InChIKey | ZBCBWPMODOFKDW-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H11NO2/c6-3-1-5-2-4-7/h5-7H,1-4H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Thioacetamide, dimethylnitrosamine and diethanolamine can induce liver damage in rats. |
| Targets | TLR | TNF-α | IL Receptor |

Diethanolamine Dilution Calculator

Diethanolamine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 9.5111 mL | 47.5556 mL | 95.1113 mL | 190.2226 mL | 237.7782 mL |
| 5 mM | 1.9022 mL | 9.5111 mL | 19.0223 mL | 38.0445 mL | 47.5556 mL |
| 10 mM | 0.9511 mL | 4.7556 mL | 9.5111 mL | 19.0223 mL | 23.7778 mL |
| 50 mM | 0.1902 mL | 0.9511 mL | 1.9022 mL | 3.8045 mL | 4.7556 mL |
| 100 mM | 0.0951 mL | 0.4756 mL | 0.9511 mL | 1.9022 mL | 2.3778 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Decanedioic acid
Catalog No.:BCN5996
CAS No.:111-20-6
- Squalene
Catalog No.:BCN5995
CAS No.:111-02-4
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tunicamycin
Catalog No.:BCC7699
CAS No.:11089-65-9
- [Sar9,Met(O2)11]-Substance P
Catalog No.:BCC6960
CAS No.:110880-55-2
- Wilforine
Catalog No.:BCN5994
CAS No.:11088-09-8
- Entrectinib
Catalog No.:BCC6410
CAS No.:1108743-60-7
- Sparfloxacin
Catalog No.:BCC4848
CAS No.:110871-86-8
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
- Cinobufotalin
Catalog No.:BCN2283
CAS No.:1108-68-5
- Garcinexanthone A
Catalog No.:BCN5993
CAS No.:1107620-67-6
- Asebotin
Catalog No.:BCN7233
CAS No.:11075-15-3
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
- Pioglitazone
Catalog No.:BCC4927
CAS No.:111025-46-8
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Ruthenium Red
Catalog No.:BCC7067
CAS No.:11103-72-3
- Annonacin
Catalog No.:BCN4734
CAS No.:111035-65-5
Diethanolamine-modified magnetic fluorescent Fe3O4@ZnS nanoparticles for ultrasensitive detection and removal of Cu2+.[Pubmed:24758043]
J Nanosci Nanotechnol. 2014 Jul;14(7):5426-9.
Currently, growing attention has been paid to the sensitive determination and removal of Cu2+ because excessive levels of Cu2+ could do harm to organisms. Herein, a novel Diethanolamine-modified magnetic fluorescent Fe3O4@ZnS nanoparticle (MFNP) for simultaneous detection and removal of Cu2+ was designed and synthesized through dithiocarbamate linkage strategy. The characterization of MFNP was confirmed by transmission electron microscope (TEM), infrared (IR) and emission spectra. The results showed that MFNP could quantificationally detect Cu2+ with high sensitivity and selectivity under a broad pH range (pH 4.5-9). The removal of Cu2+ was achieved by the aggregation-induced sedimentation (AIS) strategy and by external magnetic field.
Improving the immunostimulatory potency of diethanolamine-containing lipid A mimics.[Pubmed:23490149]
Bioorg Med Chem. 2013 Apr 15;21(8):2199-2209.
Lipid A is the active principal of gram negative bacterial lipopolysaccharide (LPS) in the activation of Toll-like receptor 4 (TLR4). Given the important role TLR4 plays in innate immunity and the development of adaptive immune responses, ligands that can modulate TLR4-mediated signaling have great therapeutic potential. Recently, we have reported a series of monophosphorylated lipid A mimics as potential ligands of TLR4, in which a Diethanolamine moiety is employed to replace the reducing end (d-glucosamine). In this paper, we describe the synthesis of two further Diethanolamine-containing lipid A mimics, 3 and 4, in an effort to mimic more closely the di-phosphate nature of natural lipid A. Both mimic 3, with an additional phosphate on the Diethanolamine acyclic scaffold, and mimic 4, with a terminal carboxylic acid moiety as a phosphate bioisostere, serve to increase the potency of the immunostimulatory response induced, as measured by the induction of the cytokines TNF-alpha, IL-6, and IL-1beta in the human monocytic cell line THP-1. In addition, mechanistic studies involving the known TLR4 antagonist lipid IVa confirm TLR4 as the target of the Diethanolamine-containing lipid A mimics.
Molecular effects of diethanolamine exposure on Calanus finmarchicus (Crustacea: Copepoda).[Pubmed:20537412]
Aquat Toxicol. 2010 Aug 15;99(2):212-22.
Alkanolamines are surface-active chemicals used in a wide range of industrial, agricultural and pharmaceutical applications and products. Of particular interest is the use of alkanolamines such as Diethanolamine (DEA) in the removal of CO(2) from natural gas and for CO(2) capture following fossil fuel combustion. Despite this widespread use, relatively little is known about the ecotoxicological impacts of these compounds. In an attempt to assess the potential effects of alkanolamines in the marine environment, a key species in the North Atlantic, the planktonic copepod Calanus finmarchicus, was studied for molecular effects following sublethal exposure to DEA. DEA-induced alterations in transcriptome and metabolome profiling were assessed using a suppression subtractive hybridization (SSH) gene library method and high resolution magic angle spinning nuclear magnetic resonance (HR-MAS NMR), respectively. Effects were observed on transcription of genes reportedly involved in lipid metabolism, antioxidant systems, metal binding, and amino acid and protein catabolism. These effects were accompanied by altered expression of fatty acid derivates, amino acids (threonine, methionine, glutamine, arginine, alanine and leucine) and cholines (choline, phosphocholine and glycerophosphocholine). Together, SSH and HR-MAS NMR offer complementary screening tools for the assessment of molecular responses of C. finmarchicus to DEA and can be used in the study of other chemicals and organisms. Concentration-response and time-response relationships between DEA exposure and single gene transcription were investigated using quantitative PCR. Specific relationships were found between DEA exposure and the transcription of genes involved in protein catabolism (ubiquitin-specific protease-7), metal ion homeostasis (ferritin) and defence against oxidative stress (gamma-glutamylcysteine synthase, glutathione synthase and Cu/Zn-superoxide dismutase). At the lowest alkanolamine concentration used in these experiments, which corresponded to 0.5% of the LC(50) concentration, no transcriptional effects were observed, giving information regarding the lower molecular effect level. Finally, similar transcription patterns were observed for a number of different genes following exposure to DEA, which indicates analogous mechanisms of toxicity and response.


