Tunicamycinantibiotic CAS# 11089-65-9 |
- Biapenem
Catalog No.:BCC1071
CAS No.:120410-24-4
- (S)-Tedizolid
Catalog No.:BCC1294
CAS No.:1431699-67-0
- Aprepitant
Catalog No.:BCC1101
CAS No.:170729-80-3
- Doripenem Hydrate
Catalog No.:BCC1160
CAS No.:364622-82-2
- Toltrazuril sulfone
Catalog No.:BCC2008
CAS No.:69004-04-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 11089-65-9 | SDF | Download SDF |
| PubChem ID | 90488851 | Appearance | Powder |
| Formula | C39H64N4O16 | M.Wt | 844.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 40 mg/mL (Need ultrasonic) | ||
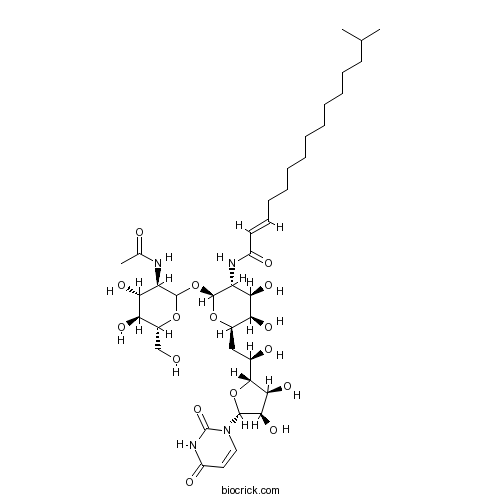
| Chemical Name | (E)-N-[(2S,3R,4R,5R,6R)-2-[(3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-6-[(2R)-2-[(2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]-2-hydroxyethyl]-4,5-dihydroxyoxan-3-yl]-14-methylpentadec-2-enamide | ||
| SMILES | CC(C)CCCCCCCCCCC=CC(=O)NC1C(C(C(OC1OC2C(C(C(C(O2)CO)O)O)NC(=O)C)CC(C3C(C(C(O3)N4C=CC(=O)NC4=O)O)O)O)O)O | ||
| Standard InChIKey | ZOCXUHJGZXXIGQ-SQXRCPDGSA-N | ||
| Standard InChI | InChI=1S/C39H64N4O16/c1-20(2)14-12-10-8-6-4-5-7-9-11-13-15-25(47)41-28-32(52)29(49)23(56-38(28)59-37-27(40-21(3)45)31(51)30(50)24(19-44)57-37)18-22(46)35-33(53)34(54)36(58-35)43-17-16-26(48)42-39(43)55/h13,15-17,20,22-24,27-38,44,46,49-54H,4-12,14,18-19H2,1-3H3,(H,40,45)(H,41,47)(H,42,48,55)/b15-13+/t22-,23-,24-,27-,28-,29+,30-,31-,32-,33+,34-,35-,36-,37?,38+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Antibiotic; inhibits GlcNAc phosphotransferase (GPT). Blocks the formation of N-glycosidic linkages by inhibiting the first step in glycoprotein synthesis. Activity induces ER stress and causes G1 arrest; can be used to induce autophagy. Tunicamycin contains four main components as follows: Homolog A, n=8, C37H60N4O16, molecular weight = 816.90 Homolog B, n=9, C38H62N4O16, molecular weight = 830.93 Homolog C, n=10, C39H64N4O16, molecular weight = 844.95 Homolog D, n=11, C40H66N4O16, molecular weight = 858.99 The composition of this product will vary from batch to batch and can be found on the relevant certificate of analysis. |

Tunicamycin Dilution Calculator

Tunicamycin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.1836 mL | 5.9179 mL | 11.8357 mL | 23.6714 mL | 29.5893 mL |
| 5 mM | 0.2367 mL | 1.1836 mL | 2.3671 mL | 4.7343 mL | 5.9179 mL |
| 10 mM | 0.1184 mL | 0.5918 mL | 1.1836 mL | 2.3671 mL | 2.9589 mL |
| 50 mM | 0.0237 mL | 0.1184 mL | 0.2367 mL | 0.4734 mL | 0.5918 mL |
| 100 mM | 0.0118 mL | 0.0592 mL | 0.1184 mL | 0.2367 mL | 0.2959 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tunicamycin (TCM or TM) [1] [2] is an antibiotic. It can block the reaction between UDP-N-acetylglucosamine and dolichol phosphate in the first step of glycoprotein synthesis and thus inhibit the synthesis of all N-linked glycoproteins, finally cause endoplasmic reticulum (ER) stress [3]. In Bacillus subtilis cells, the IC50 for TCM to inhibit the formation of dolichyl pyrophosphoryl N-acetylglucosamine (Dol-p-p-GlcNAc) is 0.03 μg/ml [2].
The ER stress response is a potent, evolutionarily conserved response to cellular metabolic stress and misfolded proteins. ER stress is induced by disruption of ER functions, such as transport to the Golgi complex or protein glycosylation, or by disturbances in the ER lumen environment, such as redox status or altered calcium homeostasis [3].
In RAW264.7 cells, tunicamycin significantly reduced LPS-induced nitrite release/production and attenuated the expression of mRNAs and hence proteins of COX-2 and iNOS. In addition, tunicamycin at a concentration of 0.5 μg/ml did not have any effect on cell survival/proliferation, but at 48h tunicamycin provided protection against activation-induced macrophage cell death. In a concentration-dependent manner, tunicamycin reduced COX-2 and iNOS protein expressions in response to LPS and induced a concurrent increase in 78-kDa glucose-regulated protein (GRP78), an ER chaperone [3].
In the small intestine of wild-type mice, tunicamycin elevated expression levels of suppressed 1370 probes and 1291 probes by >2 fold. In the small intestine of Nrf 2 (?/?) mice, tunicamycin inhibited 2024 probes and induced 3471 probes by >2 fold. Compared with results of small intestine samples, in wild-type mice liver, less well-defined genes were either suppressed (943) or elevated (750) >2 fold by tunicamycin; whereas in Nrf2 (?/?) mice liver, 3170 genes were inhibited or 39 well-defined genes were induced [1].
References:
[1]. Sujit Nair, Changjiang Xu, Guoxiang Shen, et al. Toxicogenomics of Endoplasmic Reticulum stress inducer Tunicamycin in the Small Intestine and Liver of Nrf2 Knockout and C57BL/6J Mice. Toxicol Lett., 2007, 168(1):21-39.
[2]. Masatoshi Inukai, Fujio Isono and Akira Takatsuki. Selective Inhibition of the Bacterial Translocase Reaction in Peptidoglycan Synthesis by Mureidomycins. Antimicrobial Agents and Chemotherapy, 1993, 37(5): 980-983.
[3]. Song-YiKim, Ji-SunHwang and Inn-OcHan. Tunicamycin inhibits Toll-like receptor-activated inflammation in RAW264.7 cells by suppression of NF-κB and c-Junactivity via a mechanism that is independent of ER-stress and N-glycosylation. European Journal of Pharmacology, 2013, 721: 294-300.
- [Sar9,Met(O2)11]-Substance P
Catalog No.:BCC6960
CAS No.:110880-55-2
- Wilforine
Catalog No.:BCN5994
CAS No.:11088-09-8
- Entrectinib
Catalog No.:BCC6410
CAS No.:1108743-60-7
- Sparfloxacin
Catalog No.:BCC4848
CAS No.:110871-86-8
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
- Cinobufotalin
Catalog No.:BCN2283
CAS No.:1108-68-5
- Garcinexanthone A
Catalog No.:BCN5993
CAS No.:1107620-67-6
- Asebotin
Catalog No.:BCN7233
CAS No.:11075-15-3
- beta-Escin
Catalog No.:BCC8172
CAS No.:11072-93-8
- Epimedin C
Catalog No.:BCN1040
CAS No.:110642-44-9
- Epimedin B
Catalog No.:BCN1039
CAS No.:110623-73-9
- Epimedin A
Catalog No.:BCN1038
CAS No.:110623-72-8
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Squalene
Catalog No.:BCN5995
CAS No.:111-02-4
- Decanedioic acid
Catalog No.:BCN5996
CAS No.:111-20-6
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- Oleylethanolamide
Catalog No.:BCC7084
CAS No.:111-58-0
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
Tunicamycin-induced Endoplasmic Reticulum Stress Upregulates the Expression of Pentraxin 3 in Human Retinal Pigment Epithelial Cells.[Pubmed:27980366]
Korean J Ophthalmol. 2016 Dec;30(6):468-478.
PURPOSE: To investigate the production of long pentraxin 3 (PTX3) in response to Tunicamycin-induced endoplasmic reticulum (ER) stress and its role in ER stress-associated cell death, PTX3 expression was evaluated in the human retinal pigment epithelial cell line, ARPE-19. METHODS: PTX3 production in ARPE-19 cells was analyzed in the absence or presence of Tunicamycin treatment by enzyme-linked immunosorbent assay. PTX3 protein and mRNA levels were estimated using western blot analysis and real-time reverse transcription-polymerase chain reaction, respectively. Protein and mRNA levels of CCAAT-enhancer-binding protein homologous protein (CHOP) and ARPE-19 cell viability were measured in the presence of Tunicamycin-induced ER stress in control or PTX3 small hairpin RNA (shRNA)-transfected ARPE-19 cells. RESULTS: The protein and mRNA levels of PTX3 were found to be significantly increased by Tunicamycin treatment. PTX3 production was significantly decreased in inositol-requiring enzyme 1alpha shRNA-transfected ARPE-19 cells compared to control shRNA-transfected cells. Furthermore, pretreatment with the NF-kappaB inhibitor abolished Tunicamycin-induced PTX3 production. Decreased cell viability and prolonged protein and mRNA expression of CHOP were observed under Tunicamycin-induced ER stress in PTX3 shRNA transfected ARPE-19 cells. CONCLUSIONS: These results suggest that PTX3 production increased in the presence of Tunicamycin-induced ER stress. Therefore, PTX3 could be an important protector of ER stress-induced cell death in human retinal pigment epithelial cells. Inositol-requiring enzyme 1alpha and the NF-kappaB signaling pathway may serve as potential targets for regulation of PTX3 expression in the retina. Therefore, their role in PTX3 expression needs to be further investigated.
Exendin-4 protects HUVECs from tunicamycin-induced apoptosis via inhibiting the IRE1a/JNK/caspase-3 pathway.[Pubmed:27915415]
Endocrine. 2017 Mar;55(3):764-772.
PURPOSE: The abnormal increase of apoptosis of endothelial cells induced by endoplasmic reticulum stress is a significant factor for vascular disease, especially for atherosclerosis. Protecting endothelial cells from endoplasmic reticulum stress is a crucial strategies to combate these diseases. The goal of this study was to explore the effect of Exendin-4, a glucagon-like peptide-1 receptor agonist, on Tunicamycin-induced apoptosis in human umbilical vein endothelial cells. METHODS: All studies were performed in primary human umbilical vein endothelial cells treated with Tunicamycin with or without Exendin-4 pretreatment. Markers of cell viability and apoptosis were assessed in all cells, as well as the protein expression levels of IRE1alpha (inositol requiring enzyme-1small a, Cyrillic), p-IRE1alpha, JNK (c-Jun N-terminal kinase), p-JNK, and caspase-3. RESULTS: Following Tunicamycin administration, human umbilical vein endothelial cells viability was gradually reduced in a dose-dependent manner, and fluorescence microscopy confirmed that Tunicamycin was inducing human umbilical vein endothelial cells apoptosis. This apoptotic effect was attenuated by Exendin-4 pretreatment. Similarly, the ratio of p-IRE1alpha/IRE1alpha, p-JNK/JNK and active caspase-3/procaspase-3 were increased by Tunicamycin (10 mug/ml); an effect that was counteracted by Exendin-4. The effect of exendin-4 was similar to that of the anti-endoplasmic reticulum stress agent, tauroursodeoxycholic acid (TUDCA). CONCLUSIONS: This study demonstrates that Exendin-4 can protect human umbilical vein endothelial cells from Tunicamycin-induced apoptosis. Furthermore, our data suggests that the mechanism for this effect is mediated by inhibiting the IRE1alpha/JNK/caspase-3 pathway.
MraY-antibiotic complex reveals details of tunicamycin mode of action.[Pubmed:28068312]
Nat Chem Biol. 2017 Mar;13(3):265-267.
The rapid increase of antibiotic resistance has created an urgent need to develop novel antimicrobial agents. Here we describe the crystal structure of the promising bacterial target phospho-N-acetylmuramoyl-pentapeptide translocase (MraY) in complex with the nucleoside antibiotic Tunicamycin. The structure not only reveals the mode of action of several related natural-product antibiotics but also gives an indication on the binding mode of the MraY UDP-MurNAc-pentapeptide and undecaprenyl-phosphate substrates.
Tunicamycin impairs olfactory learning and synaptic plasticity in the olfactory bulb.[Pubmed:28087337]
Neuroscience. 2017 Mar 6;344:371-379.
Tunicamycin (TM) induces endoplasmic reticulum (ER) stress and inhibits N-glycosylation in cells. ER stress is associated with neuronal death in neurodegenerative disorders, such as Parkinson's disease and Alzheimer's disease, and most patients complain of the impairment of olfactory recognition. Here we examined the effects of TM on aversive olfactory learning and the underlying synaptic plasticity in the main olfactory bulb (MOB). Behavioral experiments demonstrated that the intrabulbar infusion of TM disabled aversive olfactory learning without affecting short-term memory. Histological analyses revealed that TM infusion upregulated C/EBP homologous protein (CHOP), a marker of ER stress, in the mitral and granule cell layers of MOB. Electrophysiological data indicated that TM inhibited tetanus-induced long-term potentiation (LTP) at the dendrodendritic excitatory synapse from mitral to granule cells. A low dose of TM (250nM) abolished the late phase of LTP, and a high dose (1muM) inhibited the early and late phases of LTP. Further, high-dose, but not low-dose, TM reduced the paired-pulse facilitation ratio, suggesting that the inhibitory effects of TM on LTP are partially mediated through the presynaptic machinery. Thus, our results support the hypothesis that TM-induced ER stress impairs olfactory learning by inhibiting synaptic plasticity via presynaptic and postsynaptic mechanisms in MOB.
Primary murine airway smooth muscle cells exposed to poly(I,C) or tunicamycin synthesize a leukocyte-adhesive hyaluronan matrix.[Pubmed:19088077]
J Biol Chem. 2009 Feb 20;284(8):5299-312.
Asthmatic attacks often follow viral infections with subsequent airway smooth muscle cell proliferation and the formation of an abnormal hyaluronan extracellular matrix with infiltrated leukocytes. In this study, we show that murine airway smooth muscle cells (MASM) treated with polyinosinic acid-polycytidylic acid (poly(I,C)), a double-stranded RNA that simulates a viral infection, synthesize an abnormal hyaluronan matrix that binds leukocytes (U937 cells). Synthesis of this matrix is initiated rapidly and accumulates linearly for approximately 10 h, reaching a plateau level approximately 7-fold higher than control cultures. MASM cells treated with Tunicamycin, to induce endoplasmic reticulum stress, also rapidly initiate synthesis of the abnormal hyaluronan matrix with linear accumulation for approximately 10 h, but only reach a plateau level approximately 2-fold higher than control cultures. In contrast to poly(I,C), the response to Tunicamycin depends on cell density, with pre-confluent cells producing more abnormal matrix per cell. Furthermore, U937 cell adhesion per hyaluronan content is higher in the sparse matrix produced in response to Tunicamycin, suggesting that the structure in the poly(I,C)-induced matrix masks potential binding sites. When MASM cells were exposed to Tunicamycin and poly(I,C) at the same time, U937 cell adhesion was partially additive, implying that these two toxins stimulate hyaluronan synthesis through two different pathways. We also characterized the size of hyaluronan produced by MASM cells, in response to poly(I,C) and Tunicamycin, and we found that it ranges from 1500 to 4000 kDa, the majority of which was approximately 4000 kDa and not different in size than hyaluronan made by untreated cells.
The hepatitis B virus precore protein is retrotransported from endoplasmic reticulum (ER) to cytosol through the ER-associated degradation pathway.[Pubmed:18805786]
J Biol Chem. 2008 Nov 21;283(47):32352-60.
The hepatitis B virus precore protein is closely related to the nucleocapsid core protein but is processed distinctly in the cell and plays a different role in the viral cycle. Precore is addressed to the endoplasmic reticulum (ER) through a signal peptide, and the form present in the ER is the P22 protein. P22 is then cleaved in its C-terminal part to be secreted as HBe antigen. In addition, a cytosolic form of 22 kDa less characterized has been observed. Precore gene was shown to be implicated in viral persistence, but until now, the actual protein species involved has not been determined. Our work focuses on the cytosolic form of precore. Using human cells expressing precore and a convenient fractionation assay, we demonstrated that the cytosolic form is identical to the ER form and retrotransported in the cytoplasm through the ER-associated degradation pathway. This cellular machinery translocates misfolded proteins to the cytoplasm, where they are ubiquitinated on lysine residues and degraded by proteasome. We showed that precore escapes proteasome due to its low lysine content and accumulates in the cytosol. The role of this retrotransport was investigated. In the presence of precore, we found a specific redistribution of the Grp78/BiP chaperone protein to cytosol and demonstrated a specific interaction between precore and Grp78/BiP. Altogether, these data support the idea that the hepatitis B virus develops a strategy to take advantage of the ER-associated degradation pathway, allowing distinct subcellular localization and probably distinct roles for the viral precore protein.
Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival.[Pubmed:17135238]
J Biol Chem. 2007 Feb 16;282(7):4702-10.
Autophagy is a cellular response to adverse environment and stress, but its significance in cell survival is not always clear. Here we show that autophagy could be induced in the mammalian cells by chemicals, such as A23187, Tunicamycin, thapsigargin, and brefeldin A, that cause endoplasmic reticulum stress. Endoplasmic reticulum stress-induced autophagy is important for clearing polyubiquitinated protein aggregates and for reducing cellular vacuolization in HCT116 colon cancer cells and DU145 prostate cancer cells, thus mitigating endoplasmic reticulum stress and protecting against cell death. In contrast, autophagy induced by the same chemicals does not confer protection in a normal human colon cell line and in the non-transformed murine embryonic fibroblasts but rather contributes to cell death. Thus the impact of autophagy on cell survival during endoplasmic reticulum stress is likely contingent on the status of cells, which could be explored for tumor-specific therapy.


