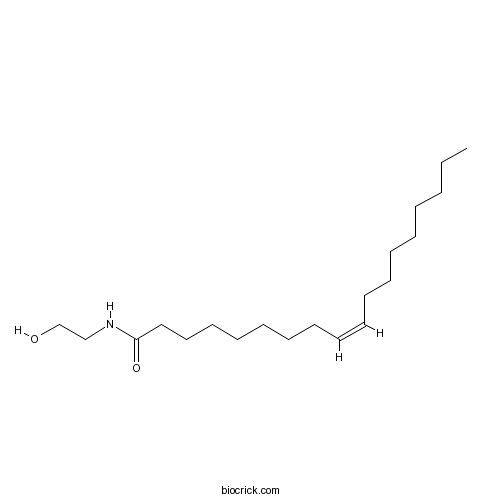
OleylethanolamideGPR55 agonist; ceramidase inhibitor CAS# 111-58-0 |
- Pyridostigmine Bromide
Catalog No.:BCC4579
CAS No.:101-26-8
- MK-0974
Catalog No.:BCC1756
CAS No.:781649-09-0
- MK-3207
Catalog No.:BCC1759
CAS No.:957118-49-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111-58-0 | SDF | Download SDF |
| PubChem ID | 5283454 | Appearance | Powder |
| Formula | C20H39NO2 | M.Wt | 325.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 20.83 mg/mL (63.99 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | (Z)-N-(2-hydroxyethyl)octadec-9-enamide | ||
| SMILES | CCCCCCCCC=CCCCCCCCC(=O)NCCO | ||
| Standard InChIKey | BOWVQLFMWHZBEF-KTKRTIGZSA-N | ||
| Standard InChI | InChI=1S/C20H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20(23)21-18-19-22/h9-10,22H,2-8,11-19H2,1H3,(H,21,23)/b10-9- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Lipid mediator and analog of anandamide that is involved in peripheral regulation of feeding. Selective GPR55 agonist (EC50 values are 0.44, >30 and >30 μM at GPR55, CB1 and CB2 respectively) and PPARα agonist (EC50 = 120 nM). Induces satiety through activation of PPARα and is also a ceramidase inhibitor. Also endogenous agonist at the GPR119 receptor. |

Oleylethanolamide Dilution Calculator

Oleylethanolamide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0719 mL | 15.3596 mL | 30.7191 mL | 61.4383 mL | 76.7978 mL |
| 5 mM | 0.6144 mL | 3.0719 mL | 6.1438 mL | 12.2877 mL | 15.3596 mL |
| 10 mM | 0.3072 mL | 1.536 mL | 3.0719 mL | 6.1438 mL | 7.6798 mL |
| 50 mM | 0.0614 mL | 0.3072 mL | 0.6144 mL | 1.2288 mL | 1.536 mL |
| 100 mM | 0.0307 mL | 0.1536 mL | 0.3072 mL | 0.6144 mL | 0.768 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Diethanolamine
Catalog No.:BCN1797
CAS No.:111-42-2
- Decanedioic acid
Catalog No.:BCN5996
CAS No.:111-20-6
- Squalene
Catalog No.:BCN5995
CAS No.:111-02-4
- PF-04620110
Catalog No.:BCC2335
CAS No.:1109276-89-2
- Tunicamycin
Catalog No.:BCC7699
CAS No.:11089-65-9
- [Sar9,Met(O2)11]-Substance P
Catalog No.:BCC6960
CAS No.:110880-55-2
- Wilforine
Catalog No.:BCN5994
CAS No.:11088-09-8
- Entrectinib
Catalog No.:BCC6410
CAS No.:1108743-60-7
- Sparfloxacin
Catalog No.:BCC4848
CAS No.:110871-86-8
- MCOPPB trihydrochloride
Catalog No.:BCC4161
CAS No.:1108147-88-1
- Cinobufotalin
Catalog No.:BCN2283
CAS No.:1108-68-5
- Garcinexanthone A
Catalog No.:BCN5993
CAS No.:1107620-67-6
- 1-Heptylamine
Catalog No.:BCN1801
CAS No.:111-68-2
- Methyl Laurate
Catalog No.:BCC8211
CAS No.:111-82-0
- Deacetylsalannin
Catalog No.:BCN4733
CAS No.:1110-56-1
- (-)-Dihydroquercetin
Catalog No.:BCN3370
CAS No.:111003-33-9
- Efonidipine hydrochloride monoethanolate
Catalog No.:BCC7767
CAS No.:111011-76-8
- 2-Amino-1-phenylethanol
Catalog No.:BCN1779
CAS No.:7568-93-6
- Muricatide
Catalog No.:BCN1780
CAS No.:111025-01-5
- Pioglitazone
Catalog No.:BCC4927
CAS No.:111025-46-8
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Ruthenium Red
Catalog No.:BCC7067
CAS No.:11103-72-3
- Annonacin
Catalog No.:BCN4734
CAS No.:111035-65-5
- 2-(2'-Hydroxy-4'-methylphenyl)propionic acid
Catalog No.:BCN7980
CAS No.:111044-84-9
Effect of High Fat Diets on Body Mass, Oleylethanolamide Plasma Levels and Oxytocin Expression in Growing Rats.[Pubmed:25976631]
J Food Sci. 2015 Jun;80(6):H1425-31.
Obesity prevalence in developed countries has promoted the need to identify the mechanisms involved in control of feeding and energy balance. We have tested the hypothesis that different fats present in diet composition may contribute in body weight gain and body indexes by regulation of oxytocin gene (oxt) expression in hypothalamus and Oleylethanolamide (OEA) levels in plasma. Sprague-Dawley rats were fed two high fat diets, based on corn (HCO) and extra virgin olive oil (HOO) and results were compared to a low fat diet (LF). LC-MS/MS analysis showed an increasing trend of OEA plasma levels in HOO group, although no significant differences were found. However, body weight gain of LF and HOO were similar and significantly lower than HCO. HCO rats also had higher Lee index than HOO. Rats fed HOO diet showed higher levels of hypothalamic oxt mRNA expression, which could indicate that oxytocin may be modulated by dietary lipids.
Oleylethanolamide activates Ras-Erk pathway and improves myocardial function in doxorubicin-induced heart failure.[Pubmed:16269455]
Endocrinology. 2006 Feb;147(2):827-34.
Oleylethanolamide (OEA) is a natural fatty acid ethanolamide produced in the heart, but its biological actions in myocardium have not yet been defined. This study was carried out to determine whether OEA could be used to prevent the development of heart failure or improve evolving heart failure. We studied in vivo and in vitro actions of OEA in cardiac muscle. In an animal model of doxorubicin cardiomyopathy, OEA showed robust effects and attenuated the progression of systolic/diastolic dysfunction and ventricular remodeling. During evolving doxorubicin cardiomyopathy, a therapeutic course of OEA treatment partially restored myocardial function. The preventive and therapeutic effects of OEA were associated with significant improvement of survival. To investigate the mechanism of OEA action in cardiac muscle, we have carried out in vitro experiments in cultured cardiomyocytes. The results showed that OEA, through activation of Ras-Raf-1-Mek-Erk signaling, inhibited doxorubicin-induced apoptosis. Additional experiments showed that OEA activation of the Erk pathway involved activation of Neu/ErbB2 receptor, which suggests OEA actions in cardiac muscle might require activation of Neu/ErbB2. In summary, OEA improved ventricular remodeling and augmented cardiac function in doxorubicin cardiomyopathy, possibly involving activation of Neu/ErbB2 and Ras-Erk signaling. These findings suggest OEA is a novel cardioprotective compound that may be used to develop new strategies for the management of cardiomyopathy.
Oleylethanolamide impairs glucose tolerance and inhibits insulin-stimulated glucose uptake in rat adipocytes through p38 and JNK MAPK pathways.[Pubmed:15886223]
Am J Physiol Endocrinol Metab. 2005 Nov;289(5):E923-9.
Oleylethanolamide (OEA) is a lipid mediator that inhibits food intake and body weight gain and also exhibits hypolipemiant actions. OEA exerts its anorectic effects peripherally through the stimulation of C-fibers. OEA is synthesized in the intestine in response to feeding, increasing its levels in portal blood after the meal. Moreover, OEA is produced by adipose tissue, and a lipolytic effect has been found. In this work, we have examined the effect of OEA on glucose metabolism in rats in vivo and in isolated adipocytes. In vivo studies showed that acute administration (30 min and 6 h) of OEA produced glucose intolerance without decreasing insulin levels. Ex vivo, we found that 10 min of preincubation with OEA inhibited 30% insulin-stimulated glucose uptake in isolated adipocytes. Maximal effect was achieved at 1 microM OEA. The related compounds palmitylethanolamide and oleic acid had no effect, suggesting a specific mechanism. Insulin-stimulated GLUT4 translocation was not affected, but OEA promoted Ser/Thr phosphorylation of GLUT4, which may impair transport activity. This phosphorylation may be partly mediated by p38 and JNK kinases, since specific inhibitors (SB-203580 and SP-600125) partly reverted the inhibitory effect of OEA on insulin-stimulated glucose uptake. These results suggest that the lipid mediator OEA inhibits insulin action in the adipocyte, impairing glucose uptake via p38 and JNK kinases, and these effects may at least in part explain the glucose intolerance produced in rats in vivo. These effects of OEA may contribute to the anorectic effects induced by this mediator, and they might be also relevant for insulin resistance in adipose tissue.
The orphan receptor GPR55 is a novel cannabinoid receptor.[Pubmed:17876302]
Br J Pharmacol. 2007 Dec;152(7):1092-101.
BACKGROUND: The endocannabinoid system functions through two well characterized receptor systems, the CB1 and CB2 receptors. Work by a number of groups in recent years has provided evidence that the system is more complicated and additional receptor types should exist to explain ligand activity in a number of physiological processes. EXPERIMENTAL APPROACH: Cells transfected with the human cDNA for GPR55 were tested for their ability to bind and to mediate GTPgammaS binding by cannabinoid ligands. Using an antibody and peptide blocking approach, the nature of the G-protein coupling was determined and further demonstrated by measuring activity of downstream signalling pathways. KEY RESULTS: We demonstrate that GPR55 binds to and is activated by the cannabinoid ligand CP55940. In addition endocannabinoids including anandamide and virodhamine activate GTPgammaS binding via GPR55 with nM potencies. Ligands such as cannabidiol and abnormal cannabidiol which exhibit no CB1 or CB2 activity and are believed to function at a novel cannabinoid receptor, also showed activity at GPR55. GPR55 couples to Galpha13 and can mediate activation of rhoA, cdc42 and rac1. CONCLUSIONS: These data suggest that GPR55 is a novel cannabinoid receptor, and its ligand profile with respect to CB1 and CB2 described here will permit delineation of its physiological function(s).
Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha.[Pubmed:12955147]
Nature. 2003 Sep 4;425(6953):90-3.
Oleylethanolamide (OEA) is a naturally occurring lipid that regulates satiety and body weight. Although structurally related to the endogenous cannabinoid anandamide, OEA does not bind to cannabinoid receptors and its molecular targets have not been defined. Here we show that OEA binds with high affinity to the peroxisome-proliferator-activated receptor-alpha (PPAR-alpha), a nuclear receptor that regulates several aspects of lipid metabolism. Administration of OEA produces satiety and reduces body weight gain in wild-type mice, but not in mice deficient in PPAR-alpha. Two distinct PPAR-alpha agonists have similar effects that are also contingent on PPAR-alpha expression, whereas potent and selective agonists for PPAR-gamma and PPAR-beta/delta are ineffective. In the small intestine of wild-type but not PPAR-alpha-null mice, OEA regulates the expression of several PPAR-alpha target genes: it initiates the transcription of proteins involved in lipid metabolism and represses inducible nitric oxide synthase, an enzyme that may contribute to feeding stimulation. Our results, which show that OEA induces satiety by activating PPAR-alpha, identify an unexpected role for this nuclear receptor in regulating behaviour, and raise possibilities for the treatment of eating disorders.
Antinociceptive activity of the endogenous fatty acid amide, palmitylethanolamide.[Pubmed:11426841]
Eur J Pharmacol. 2001 May 11;419(2-3):191-8.
The endogenous fatty acid ethanolamide, palmitylethanolamide, alleviated, in a dose-dependent manner, pain behaviors elicited in mice by injections of formalin (5%, intraplantar), acetic acid (0.6%, 0.5 ml per animal, intraperitoneal, i.p.), kaolin (2.5 mg per animal, i.p.), and magnesium sulfate (120 mg per kg, i.p.). The antinociceptive effects of palmitylethanolamide were prevented by the cannabinoid CB2 receptor antagonist SR144528 [N-([1s]-endo-1.3.3-trimethylbicyclo[2.3.1]heptan-2-yl)-5-(4-chloro-3-methylpheny l)-1-(4-methylbenzyl)-pyrazole-3-carboxamide], not by the cannabinoid CB1 receptor antagonist SR141716A [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazol e-3-carboxamide x HCl]. By contrast, palmitylethanolamide had no effect on capsaicin-evoked pain behavior or thermal nociception. The endogenous cannabinoid, anandamide (arachidonylethanolamide), alleviated nociception in all tests (formalin, acetic acid, kaolin, magnesium sulfate, capsaicin and hot plate). These effects were prevented by the cannabinoid CB1 receptor antagonist SR141716A, not the cannabinoid CB2 receptor antagonist SR141716A. Additional fatty acid ethanolamides (Oleylethanolamide, myristylethanolamide, palmitOleylethanolamide, palmitelaidylethanolamide) had little or no effect on formalin-evoked pain behavior, and were not investigated in other pain models. These results support the hypothesis that endogenous palmitylethanolamide participates in the intrinsic control of pain initiation. They also suggest that the putative receptor site activated by palmitylethanolamide may provide a novel target for peripherally acting analgesic drugs.
An anorexic lipid mediator regulated by feeding.[Pubmed:11700558]
Nature. 2001 Nov 8;414(6860):209-12.
Oleylethanolamide (OEA) is a natural analogue of the endogenous cannabinoid anandamide. Like anandamide, OEA is produced in cells in a stimulus-dependent manner and is rapidly eliminated by enzymatic hydrolysis, suggesting a function in cellular signalling. However, OEA does not activate cannabinoid receptors and its biological functions are still unknown. Here we show that, in rats, food deprivation markedly reduces OEA biosynthesis in the small intestine. Administration of OEA causes a potent and persistent decrease in food intake and gain in body mass. This anorexic effect is behaviourally selective and is associated with the discrete activation of brain regions (the paraventricular hypothalamic nucleus and the nucleus of the solitary tract) involved in the control of satiety. OEA does not affect food intake when injected into the brain ventricles, and its anorexic actions are prevented when peripheral sensory fibres are removed by treatment with capsaicin. These results indicate that OEA is a lipid mediator involved in the peripheral regulation of feeding.
Differential regulation of sphingomyelinase and ceramidase activities by growth factors and cytokines. Implications for cellular proliferation and differentiation.[Pubmed:7559485]
J Biol Chem. 1995 Oct 6;270(40):23305-9.
Sphingosine is a product of sphingolipid metabolism that has been linked to a protein kinase C-independent mitogenic response. In previously published data, utilizing an in vitro model system for platelet-derived growth factor (PDGF)-induced vascular smooth muscle proliferation, we have demonstrated that sphingosine is increased at the expense of a concomitant decrease in ceramide formation, implicating an altered ceramidase activity. To explore mechanisms of growth factor-stimulated sphingosine formation, we have developed and investigated a cell-free model system assessing ceramidase activity. We now report that an alkaline, membrane-associated, ceramidase activity in the rat glomerular mesangial cell, a smooth muscle-like pericyte, is up-regulated by growth factors, apparently via a tyrosine kinase phosphorylation mechanism. PDGF also stimulated sphingomyelinase activity which generates sufficient substrate to drive the subsequent ceramidase reaction. Inflammatory cytokines, including interleukin-1, and tumor necrosis factor-alpha, stimulated sphingomyelinase but not ceramidase activity, a result consistent with the cellular accumulation of the ceramide, apoptidic, differentiating second messenger. Mitogenic vasoconstrictor peptides such as endothelin-1 stimulated neither sphingomyelinase nor ceramidase activities. An inhibitor of ceramidase activity, N-oleoylethanolamine, reduced PDGF- but not endothelin-1-stimulated proliferation. Thus, we conclude that, in mesangial cells, growth factors but not vasoconstrictor peptides or cytokines induce mitogenesis, in part, through ceramidase-mediated sphingosine formation.


