NF 110Potent P2X3 antagonist CAS# 111150-22-2 |
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- Entinostat (MS-275,SNDX-275)
Catalog No.:BCC3595
CAS No.:209783-80-2
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 111150-22-2 | SDF | Download SDF |
| PubChem ID | 16066783 | Appearance | Powder |
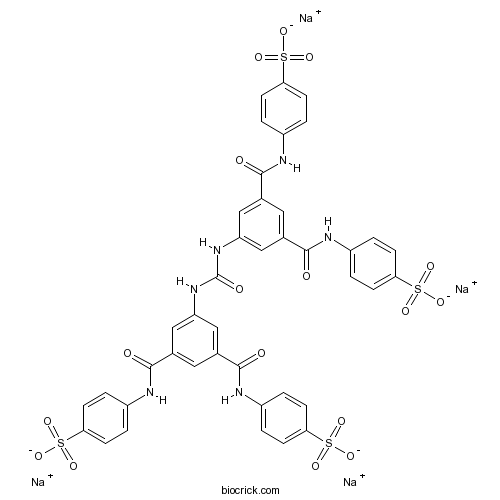
| Formula | C41H28N6Na4O17S4 | M.Wt | 1096.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 40 mM in water | ||
| Chemical Name | 4,4',4'',4'''-[Carbonylbis[imino-5,1,3-be | ||
| SMILES | C1=CC(=CC=C1NC(=O)C2=CC(=CC(=C2)NC(=O)NC3=CC(=CC(=C3)C(=O)NC4=CC=C(C=C4)S(=O)(=O)[O-])C(=O)NC5=CC=C(C=C5)S(=O)(=O)[O-])C(=O)NC6=CC=C(C=C6)S(=O)(=O)[O-])S(=O)(=O)[O-].[Na+].[Na+].[Na+].[Na+] | ||
| Standard InChIKey | AQJHZNCSXLBXMY-UHFFFAOYSA-J | ||
| Standard InChI | InChI=1S/C41H32N6O17S4.4Na/c48-37(42-27-1-9-33(10-2-27)65(53,54)55)23-17-24(38(49)43-28-3-11-34(12-4-28)66(56,57)58)20-31(19-23)46-41(52)47-32-21-25(39(50)44-29-5-13-35(14-6-29)67(59,60)61)18-26(22-32)40(51)45-30-7-15-36(16-8-30)68(62,63)64;;;;/h1-22H,(H,42,48)(H,43,49)(H,44,50)(H,45,51)(H2,46,47,52)(H,53,54,55)(H,56,57,58)(H,59,60,61)(H,62,63,64);;;;/q;4*+1/p-4 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | High affinity P2X3 receptor antagonist (Ki values are 36, 82 and 4144 nM for P2X3, P2X1 and P2X2 recombinant receptors respectively). Shows no activity at P2Y1, P2Y2 and P2Y11 receptors (IC50 > 10 μM). Potently inhibits α,β-meATP-evoked desensitizing currents in rat DRG neurons (IC50 = 527 nM). Shows antitumor activity against several tumor types. |

NF 110 Dilution Calculator

NF 110 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 0.9117 mL | 4.5583 mL | 9.1166 mL | 18.2332 mL | 22.7915 mL |
| 5 mM | 0.1823 mL | 0.9117 mL | 1.8233 mL | 3.6466 mL | 4.5583 mL |
| 10 mM | 0.0912 mL | 0.4558 mL | 0.9117 mL | 1.8233 mL | 2.2792 mL |
| 50 mM | 0.0182 mL | 0.0912 mL | 0.1823 mL | 0.3647 mL | 0.4558 mL |
| 100 mM | 0.0091 mL | 0.0456 mL | 0.0912 mL | 0.1823 mL | 0.2279 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 14-Hydroxy sprengerinin C
Catalog No.:BCN2777
CAS No.:1111088-89-1
- FERb 033
Catalog No.:BCC7701
CAS No.:1111084-78-6
- Dacomitinib (PF299804, PF299)
Catalog No.:BCC3683
CAS No.:1110813-31-4
- Fmoc-Ser(Trt)-OH
Catalog No.:BCC3546
CAS No.:111061-56-4
- Fmoc-D-Lys(Trt)-OH
Catalog No.:BCC2594
CAS No.:111061-54-2
- N-Benzoyl-2-hydroxy-2-phenylethylamine
Catalog No.:BCN1622
CAS No.:111059-46-2
- Ginkgolic acid C17:1
Catalog No.:BCN5334
CAS No.:111047-30-4
- 2-(2'-Hydroxy-4'-methylphenyl)propionic acid
Catalog No.:BCN7980
CAS No.:111044-84-9
- Annonacin
Catalog No.:BCN4734
CAS No.:111035-65-5
- Ruthenium Red
Catalog No.:BCC7067
CAS No.:11103-72-3
- Vitamin A2
Catalog No.:BCC8367
CAS No.:11103-57-4
- Pioglitazone
Catalog No.:BCC4927
CAS No.:111025-46-8
- PF-04880594
Catalog No.:BCC3998
CAS No.:1111636-35-1
- 1,2-O-Dilinoleoyl-3-O-beta-D-galactopyranosylracglycerol
Catalog No.:BCN6768
CAS No.:111187-15-6
- 7,3',4'-Trihydroxy-3-benzyl-2H-chromene
Catalog No.:BCN1621
CAS No.:1111897-60-9
- CGS 20625
Catalog No.:BCC7375
CAS No.:111205-55-1
- Episappanol
Catalog No.:BCN7940
CAS No.:111254-18-3
- Sappanol
Catalog No.:BCN3735
CAS No.:111254-19-4
- Lestaurtinib
Catalog No.:BCC2440
CAS No.:111358-88-4
- Zileuton
Catalog No.:BCC2515
CAS No.:111406-87-2
- Azadirachtin
Catalog No.:BCC8123
CAS No.:11141-17-6
- Pinocembrin diacetate
Catalog No.:BCN5997
CAS No.:111441-88-4
- Axinysone B
Catalog No.:BCN7713
CAS No.:1114491-60-9
- Naltrindole hydrochloride
Catalog No.:BCC6773
CAS No.:111469-81-9
Transactivation of the human cdc2 promoter by adenovirus E1A. E1A induces the expression and assembly of a heteromeric complex consisting of the CCAAT box binding factor, CBF/NF-Y, and a 110-kDa DNA-binding protein.[Pubmed:10438472]
J Biol Chem. 1999 Aug 13;274(33):23043-51.
Cyclin-dependent kinases (CDKs) play an important role in the eukaryotic cell cycle progression. Cdc2 (CDK1) is expressed in late G(1)/S phase and required for G(2) to M phase transition in higher eukaryotes. The oncoproteins, SV40 large T antigen and adenovirus E1A, induce a 110-kDa protein which specifically recognizes the two inverted CCAAT motifs of the cdc2 promoter in cycling cells and plays an essential role in transactivation of the human cdc2 promoter. Since these CCAAT motifs also conform to the consensus binding sites for the ubiquitous heterotrimeric transcription factor, CBF/NF-Y, the role of CBF/NF-Y in the transactivation of the cdc2 promoter was examined in this study. Our results indicate that CBF/NF-Y and the 110-kDa protein interact with the CCAAT box motif to form a heteromeric complex. However, mutagenesis of the pentanucleotide CCAAT motif or in the presence of urea greater than 2.5 M, no heteromeric complex was formed. In contrast, the 110-kDa protein could still bind the mutant CCAAT motif or with the wild type motif in the presence of 2.5 M urea. Furthermore, E1A.12S induced the gene expression of all three subunits of CBF/NF-Y. Coexpression of E1A and a dominant negative mutant NF-YA subunit significantly reduced the E1A-mediated transactivation of the cdc2 promoter in a dose-dependent manner. These results support the conclusion that E1A protein mediates optimal transactivation of the human cdc2 promoter by inducing the expression and assembly of a heteromeric complex consisting of the 110-kDa protein and the CBF/NF-Y which interacts with the two CCAAT motifs of the cdc2 promoter.
The suramin analog 4,4',4'',4'''-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors: subtype selectivity is determined by location of sulfonic acid groups.[Pubmed:16551782]
Mol Pharmacol. 2006 Jun;69(6):2058-67.
We have previously identified the suramin analog 4,4',4'',4'''-(carbonylbis(imino-5,1,3-benzenetriylbis(carbonylimino)))tetrakis-b enzene-1,3-disulfonic acid (NF449) as a low nanomolar potency antagonist of recombinant P2X(1) receptors. Here, we characterize, by two-electrode voltage-clamp electrophysiology, three isomeric suramin analogs designated para-4,4',4'',4''''-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetrakis-benzenesulfonic acid (NF110), meta-(3,3',3'',3''''-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF448), and ortho-(2,2',2'',2''''-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (MK3) with respect to their potency in antagonizing rat P2X receptor-mediated inward currents in Xenopus laevis oocytes. Meta, para, and ortho refer to the position of the single sulfonic acid group relative to the amide bond linking the four symmetrically oriented benzenesulfonic acid moieties to the central, invariant suramin core. NF448, NF110, and MK3 were >200-fold less potent in blocking P2X(1) receptors than NF449, from which they differ structurally only by having one instead of two sulfonic acid residues per benzene ring. Although the meta- and ortho-isomers retained P2X(1) receptor selectivity, the para-isomer NF110 exhibited a significantly increased activity at P2X(3) receptors (K(i) approximately 36 nM) and displayed the following unique selectivity profile among suramin derivatives: P2X(2+3) = P2X(3) > P2X(1) > P2X(2) >> P2X(4) > P2X(7). The usefulness of NF110 as a P2X(3) receptor antagonist in native tissues could be demonstrated by showing that NF110 blocks alphabeta-methylene-ATP-induced currents in rat dorsal root ganglia neurons with similar potency as recombinant rat P2X(3) receptors. Together, these data highlight the importance of both the number and exact location of negatively charged groups for P2X subtype potency and selectivity.
Structure-activity relationships of analogues of NF449 confirm NF449 as the most potent and selective known P2X1 receptor antagonist.[Pubmed:15072843]
Eur J Med Chem. 2004 Apr;39(4):345-57.
NF449 [4,4',4",4"'-(carbonylbis(imino-5,1,3-benzenetriyl-bis(carbonylimino)))tetrakisbe nzene-1,3-disulfonic acid-octasodiumsalt)] was recently described to inhibit recombinant rP2X(1) receptors (Naunyn Schmiedeberg's Arch. Pharmacol. 364 (2001) 285). The purpose of this study was to examine structure-activity-relationships at P2 receptors of a series of NF449 analogues. Thus, compounds containing various arylaminemono-, di-, or trisulfonic acids and a replacement of the central urea bridge were synthesized. NF449 displayed a pIC(50) at P2X(1) receptors (rat vas deferens) of 6.31 +/- 0.04 being at least 19-fold more potent at P2X(1) than at P2X(3), P2Y(1), P2Y(2), or P2Y(11). Any deletion or change of position of sulfonic acid groups or replacing the central urea bond by the bisamide of terephthalic acid reduced the potency at P2X(1) by at least 90%. All compounds were very weak antagonists at P2Y(2) or P2Y(11) receptors (pIC(50) < 4.5). In conclusion, NF449 remains the most potent and selective P2X(1) antagonist known. Potential lead compounds among the suramin class for P2X(3) (16d) and P2Y(1) (16a) receptors were identified.
Antitumour activity of suramin analogues in human tumour cell lines and primary cultures of tumour cells from patients.[Pubmed:10762755]
Eur J Cancer. 2000 Apr;36(6):803-9.
Suramin has shown promising antitumour activity against several tumour types, both in vitro and in vivo, but the clinical utility of this compound is hampered by its unfavourable toxicity profile. In the present study, the semi-automated fluorometric microculture cytotoxicity assay (FMCA) was employed for evaluation of the cytotoxicity of seven suramin analogues in vitro in a panel of human tumour cell lines and in primary cultures of tumour cells from patients. Like suramin, the analogues showed little sensitivity to resistance mechanisms involving P-glycoprotein, topoisomerase II, multidrug resistance associated protein and glutathione-mediated drug resistance. In the cell line panel, NF067 and FCE 26644 showed activity comparable with suramin. All analogues were less potent than suramin in patient cells except for FCE 26644. Correlation to suramin activity patterns in the cell line panel was highest for NF037 and low to moderate for the remaining analogues. In patient cells, high correlation coefficients were obtained for FCE 26644, NF110, NF031 and NF037. The results indicate that the cytotoxic activity of suramin on patient tumour cells is shared by the analogues with FCE 26644 being the most active. The pharmacophore for cytotoxicity in patient cells may be different from that observed in the cell lines.


