EPZ015666PRMT5 inhibitor CAS# 1616391-65-1 |
- 3-Deazaneplanocin,DZNep
Catalog No.:BCC1129
CAS No.:102052-95-9
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1616391-65-1 | SDF | Download SDF |
| PubChem ID | 90241673 | Appearance | Powder |
| Formula | C20H25N5O3 | M.Wt | 383.44 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (260.80 mM; Need ultrasonic) | ||
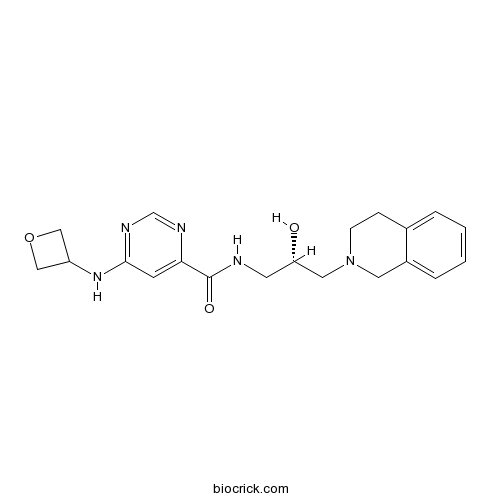
| Chemical Name | N-[(2S)-3-(3,4-dihydro-1H-isoquinolin-2-yl)-2-hydroxypropyl]-6-(oxetan-3-ylamino)pyrimidine-4-carboxamide | ||
| SMILES | C1CN(CC2=CC=CC=C21)CC(CNC(=O)C3=CC(=NC=N3)NC4COC4)O | ||
| Standard InChIKey | ZKXZLIFRWWKZRY-KRWDZBQOSA-N | ||
| Standard InChI | InChI=1S/C20H25N5O3/c26-17(10-25-6-5-14-3-1-2-4-15(14)9-25)8-21-20(27)18-7-19(23-13-22-18)24-16-11-28-12-16/h1-4,7,13,16-17,26H,5-6,8-12H2,(H,21,27)(H,22,23,24)/t17-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | EPZ015666 is an orally available inhibitor of PRMT5 enzymatic activity in biochemical assays with IC50 of 22 nM and broad selectivity against a panel of other histone methyltransferases.In Vitro:Treatment of MCL cell lines with EPZ015666 leads to inhibition of SmD3 methylation and cell death, with IC50 values in the nanomolar range[1]. EPZ015666, a potent peptide-competitive and SAM-cooperative inhibitor with >10,000-fold specificity against PRMT5 relative to other methyltransferases[2].In Vivo:EPZ015666 is orally bioavailable and amenable to in vivo studies. We performed 21-d efficacy studies in severe combined immunodeficiency (SCID) mice bearing subcutaneous Z-138 and Maver-1 xenografts, with twice-daily (BID) oral dosing on four dose groups: 25, 50, 100 and 200 mg per kilogram of body weight (mg/kg). After 21 d of continuous dosing, animals are euthanized, and blood and tissues are analyzed to determine the relationship between methyl-mark pharmacodynamics and tumor-growth inhibition (TGI). EPZ015666 showed dose-dependent exposure and TGI after 21 d in both MCL models. Tumors in all EPZ015666 dose groups measured on day 21 showed statistically significant differences in weight, volume and tumor growth compared to vehicle-treated tumors. Dosing at 200 mg/kg BID induced tumor stasis in Z-138 cells, with >93% TGI after 21 d, whereas Maver-1 cells showed >70% TGI. Additionally, a third MCL xenograft is tested using the Granta-519 cell line, a fast-growing model that reached endpoint on day 18 and showed dose-dependent efficacy with 45% TGI in the 200 mg/kg group. EPZ015666 is well tolerated in all three models, with minimal bodyweight loss in the 200 mg/kg dose group and no other clinical observations[1]. References: | |||||

EPZ015666 Dilution Calculator

EPZ015666 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.608 mL | 13.0398 mL | 26.0797 mL | 52.1594 mL | 65.1992 mL |
| 5 mM | 0.5216 mL | 2.608 mL | 5.2159 mL | 10.4319 mL | 13.0398 mL |
| 10 mM | 0.2608 mL | 1.304 mL | 2.608 mL | 5.2159 mL | 6.5199 mL |
| 50 mM | 0.0522 mL | 0.2608 mL | 0.5216 mL | 1.0432 mL | 1.304 mL |
| 100 mM | 0.0261 mL | 0.1304 mL | 0.2608 mL | 0.5216 mL | 0.652 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
EPZ015666 is a potent, selective and orally bioavailable PRMT5 inhibitor with Ki of 5 nM, >20,000-fold selectivity over other PMTs.
- VRT-1353385
Catalog No.:BCC6433
CAS No.:1616113-45-1
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- Erythrinin D
Catalog No.:BCN6858
CAS No.:1616592-59-6
- 1,5-difluoro-3-methyl-2-nitrobenzene
Catalog No.:BCN6404
CAS No.:1616526-80-7
- Erythrinin G
Catalog No.:BCN6857
CAS No.:1616592-61-0
- Erythrinin H
Catalog No.:BCN6870
CAS No.:1616592-62-1
- ONC201
Catalog No.:BCC3989
CAS No.:1616632-77-9
- Dodovisone A
Catalog No.:BCN6839
CAS No.:1616683-50-1
- Dodovisone B
Catalog No.:BCN6867
CAS No.:1616683-51-2
- Dodovisone C
Catalog No.:BCN6872
CAS No.:1616683-52-3
- Dodovisone D
Catalog No.:BCN6871
CAS No.:1616683-53-4
- Dodovislactone A
Catalog No.:BCN7399
CAS No.:1616683-54-5
- Dodovislactone B
Catalog No.:BCN7398
CAS No.:1616683-55-6
- Caesalpine A
Catalog No.:BCN7376
CAS No.:1616757-59-5
Species differences in metabolism of EPZ015666, an oxetane-containing protein arginine methyltransferase-5 (PRMT5) inhibitor.[Pubmed:26294260]
Xenobiotica. 2016;46(3):268-77.
1. Metabolite profiling and identification studies were conducted to understand the cross-species differences in the metabolic clearance of EPZ015666, a first-in-class protein arginine methyltransferase-5 (PRMT5) inhibitor, with anti-proliferative effects in preclinical models of Mantle Cell Lymphoma. EPZ015666 exhibited low clearance in human, mouse and rat liver microsomes, in part by introduction of a 3-substituted oxetane ring on the molecule. In contrast, a higher clearance was observed in dog liver microsomes (DLM) that translated to a higher in vivo clearance in dog compared with rodent. 2. Structure elucidation via high resolution, accurate mass LC-MS(n) revealed that the prominent metabolites of EPZ015666 were present in hepatocytes from all species, with the highest turnover rate in dogs. M1 and M2 resulted from oxidative oxetane ring scission, whereas M3 resulted from loss of the oxetane ring via an N-dealkylation reaction. 3. The formation of M1 and M2 in DLM was significantly abrogated in the presence of the specific CYP2D inhibitor, quinidine, and to a lesser extent by the CYP3A inhibitor, ketoconazole, corroborating data from human recombinant isozymes. 4. Our data indicate a marked species difference in the metabolism of the PRMT5 inhibitor EPZ015666, with oxetane ring scission the predominant metabolic pathway in dog mediated largely by CYP2D.
Structure and Property Guided Design in the Identification of PRMT5 Tool Compound EPZ015666.[Pubmed:26985292]
ACS Med Chem Lett. 2015 Dec 2;7(2):162-6.
The recent publication of a potent and selective inhibitor of protein methyltransferase 5 (PRMT5) provides the scientific community with in vivo-active tool compound EPZ015666 (GSK3235025) to probe the underlying pharmacology of this key enzyme. Herein, we report the design and optimization strategies employed on an initial hit compound with poor in vitro clearance to yield in vivo tool compound EPZ015666 and an additional potent in vitro tool molecule EPZ015866 (GSK3203591).


