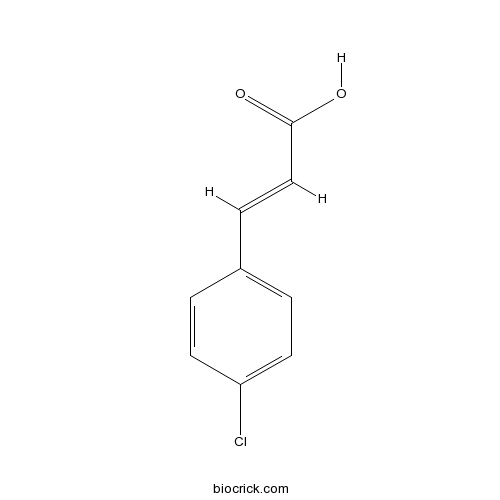
4-Chlorocinnamic acidCAS# 1615-02-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1615-02-7 | SDF | Download SDF |
| PubChem ID | 637797 | Appearance | Cryst. |
| Formula | C9H7ClO2 | M.Wt | 182.60 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (E)-3-(4-chlorophenyl)prop-2-enoic acid | ||
| SMILES | C1=CC(=CC=C1C=CC(=O)O)Cl | ||
| Standard InChIKey | GXLIFJYFGMHYDY-ZZXKWVIFSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4-Chlorocinnamic acid is a photosensitive compound. 2-Chlorocinnamic acid and 4-chlorocinnamic acid show potent urease inhibitory activities with the respective IC50 values of 0.66 and 1.10 uM. |
| In vitro | Photosensitive semiconductor nanocrystals, photosensitive composition comprising semiconductor nanocrystals and method for forming semiconductor nanocrystal pattern using the same[Reference: WebLink]US 8758864 B2[P]. 2014.4. The organic-inorganic hybrid electroluminescent device according to claim 1, wherein the compound containing a photosensitive functional group is selected from a group consisting of methacrylic acid, crotonic acid, vinylacetic acid, tiglic acid, 3,3-dimethylacrylic acid, trans-2-pentenoic acid, 4-pentenoic acid, trans-2-methyl-2-pentenoic acid, 2,2-dimethyl-4-pentenoic acid, trans-2-hexenoic acid, trans-3-hexenoic acid, 2-ethyl-2-hexenoic acid, 6-heptenoic acid, 2-octenoic acid, citronellic acid, undecylenic acid, myristoleic acid, palmitoleic acid, oleic acid, elaidic acid, cis-11-elcosenoic acid, euric acid, nervonic acid, trans-2,4-pentadienoic acid, 2,4-hexadienoic acid, 2,6-heptadienoic acid, geranic acid, linoleic acid, 11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, arachidonic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, fumaric acid, maleic acid, itaconic acid, ciraconic acid, mesaconic acid, trans-glutaconic acid, trans-beta-hydromuconic acid, trans-traumatic acid, trans-muconic acid, cis-aconitic acid, trans-aconitic acid, cis-3-chloroacrylic acid, trans-3-chloroacrylic acid, 2-bromoacrylic acid, 2-(trifluoromethyl)acryl-ic acid, trans-styrylacetic acid, trans-cinnamic acid, alpha.-methylcinnamic acid, 2-methylcinnamic acid, 2-fluorocinnamic acid, 2-(trifluoromethyl)cinnamic acid, 2-chlorocinnamic acid, 2-methoxycinnamic acid, 2-hydroxycinnamic acid, 2-nitrocinnamic acid, 2-carboxycinnamic acid, trans-3-fluorocinnamic acid, 3-(trifluoromethyl)cinnamic acid, 3-chlorocinnamic acid, 3-bromocinnamic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 3-nitrocinnamic acid, 4-Methylcinnamic acid, 4-fluorocinnamic acid, trans-4-(trifluoromethyl)-cinnamic acid, 4-Chlorocinnamic acid, 4-bromocinnamic acid, 4-methoxycinnamic acid, 4-hydroxycinnamic acid, 4-nitrocinnamic acid, 3,3-dimethoxycinnamic acid, 4-vinylbenzoic acid, allyl methyl sulfide, allyl disulfide, diallyl amine, oleylamine, 3-amino-1-propanol vinyl ether, 4-chlorocinnamonitrile, 4-methoxycinnamonitrile, 3,4-dimethoxycinnamonitrile, 4-dimethylaminocinnamonitrile, acrylonitrile, allyl cyanide, crotononitrile, methacrylonitrile, cis-2-pentenenitrile, trans-3-pentenenitrile, 3,7-dimethyl-2,6-octadienenitrile, and 1,4-dicyano-2-butene. |
| Kinase Assay | Synthesis, Characterization and Biological Evaluation of Two Silver(I) trans‐Cinnamate Complexes as Urease Inhibitors[Reference: WebLink]Zeitschrift Für Anorganische Und Allgemeine Chemie,2014, 640(2):423-8.Two new silver(I) trans‐cinnamates, namely [Ag(2‐cca)(H2O)]2 (1) and [Ag(4‐cca)] n (2) (2‐ccaH = 2‐chlorocinnamic acid and 4‐ccaH = 4-Chlorocinnamic acid ), were synthesized and structurally characterized. |
| Structure Identification | CrystEngComm.2012 Aug3;14(22):7792-7799.Dependence of the solid-state photomechanical response of 4-chlorocinnamic acid on crystal shape and size.[Reference: WebLink]The photochemical dynamics of crystals composed of 4-Chlorocinnamic acid (4Cl-CA), whose photochemistry is dominated by an irreversible [2+2] photodimerization reaction, are studied using 13C solid-state NMR, powder X-ray diffraction, and optical and electron microscopy. |

4-Chlorocinnamic acid Dilution Calculator

4-Chlorocinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.4765 mL | 27.3823 mL | 54.7645 mL | 109.529 mL | 136.9113 mL |
| 5 mM | 1.0953 mL | 5.4765 mL | 10.9529 mL | 21.9058 mL | 27.3823 mL |
| 10 mM | 0.5476 mL | 2.7382 mL | 5.4765 mL | 10.9529 mL | 13.6911 mL |
| 50 mM | 0.1095 mL | 0.5476 mL | 1.0953 mL | 2.1906 mL | 2.7382 mL |
| 100 mM | 0.0548 mL | 0.2738 mL | 0.5476 mL | 1.0953 mL | 1.3691 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- SIN-1 chloride
Catalog No.:BCC5670
CAS No.:16142-27-1
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- H-Orn(Z)-OtBu.HCl
Catalog No.:BCC2677
CAS No.:161234-80-6
- Cimicidanol 3-O-alpha-L-arabinoside
Catalog No.:BCN6528
CAS No.:161207-05-2
- H-Asp(OEt)-OEt.HCl
Catalog No.:BCC2888
CAS No.:16115-68-7
- Bidwillol A
Catalog No.:BCN4858
CAS No.:161099-42-9
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
- VRT-1353385
Catalog No.:BCC6433
CAS No.:1616113-45-1
- EPZ015666
Catalog No.:BCC5653
CAS No.:1616391-65-1
Probing the Crystal Structure Landscape by Doping: 4-Bromo, 4-Chloro, and 4-Methylcinnamic Acids.[Pubmed:29893027]
Angew Chem Int Ed Engl. 2018 Jul 20;57(30):9279-9283.
Accessing the data points in the crystal structure landscape of a molecule is a challenging task, either experimentally or computationally. We have charted the crystal structure landscape of 4-bromocinnamic acid (4BCA) experimentally and computationally: experimental doping is achieved with 4-methylcinnamic acid (4MCA) to obtain new crystal structures; computational doping is performed with 4-Chlorocinnamic acid (4CCA) as a model system, because of the difficulties associated in parameterizing the Br atom. The landscape of 4CCA is explored experimentally in turn, also by doping it with 4MCA, and is found to bear a close resemblance to the landscape of 4BCA, justifying the ready miscibility of these two halogenated cinnamic acids to form solid solutions without any change in crystal structure. In effect, 4MCA, 4CCA and 4BCA form a commutable group of crystal structures, which may be realized experimentally or computationally, and constitute the landscape. Unlike the results obtained by Kitaigorodskii, all but two of the multiple solid solutions obtained in the methyl-doping experiments take structures that are different from the hitherto observed crystal forms of the parent compounds. Even granted that the latter might be inherently polymorphic, this unusual observation provokes the suggestion that solid solution formation may be used to probe the crystal structure landscape. The influence of pipi interactions, weak hydrogen bonds and halogen bonds in directing the formation of these new structures is also seen.
Support for Natural Small-Molecule Phenols as Anxiolytics.[Pubmed:29210995]
Molecules. 2017 Dec 6;22(12). pii: molecules22122138.
Natural small-molecule phenols (NSMPs) share some bioactivities. The anxiolytic activity of NSMPs is attracting attention in the scientific community. This paper provides data supporting the hypothesis that NSMPs are generally anxiolytic. The anxiolytic activities of seven simple phenols, including phloroglucinol, eugenol, protocatechuic aldehyde, vanillin, thymol, ferulic acid, and caffeic acid, were assayed with the elevated plus maze (EPM) test in mice. The oral doses were 5, 10 and 20 mg/kg, except for phloroglucinol for which the doses were 2.5, 5 and 10 mg/kg. All tested phenols had anxiolytic activity in mice. The phenolic hydroxyl group in 4-hydroxycinnamic acid (4-OH CA) was essential for the anxiolytic activity in the EPM test in mice and rats compared to 4-Chlorocinnamic acid (4-Cl CA). The in vivo spike recording of rats' hippocampal neurons also showed significant differences between 4-OH CA and 4-Cl CA. Behavioral and neuronal spike recording results converged to indicate the hippocampal CA1 region might be a part of the anxiolytic pathways of 4-OH CA. Therefore, our study provides further experimental data supporting NSMPs sharing anxiolytic activity, which may have general implications for phytotherapy because small phenols occur extensively in herbal medicines.
Investigation of the removal mechanism of antibiotic ceftazidime by green algae and subsequent microbic impact assessment.[Pubmed:28646154]
Sci Rep. 2017 Jun 23;7(1):4168.
The present study provides an integrated view of algal removal of the antibiotic ceftazidime and its basic parent structure 7-aminocephalosporanic acid (7-ACA), including contribution analysis, bacteriostatic and aquatic toxic assessment and metabolite verification. 92.70% and 96.07% of the two target compounds was removed after the algal treatment, respectively. The algal removal can be separated into three steps: a rapid adsorption, a slow cell wall-transmission and the final biodegradation. Additionally, while ceftazidime demonstrated an excellent inhibitory effect on Escherichia coli, there was no bacteriostasis introduced after the algal treatment, which could avoid favoring the harmful selective pressure. On the other hand, no significant aquatic impact of the two target compounds on rotifers was observed and it was not enhanced after the algal treatment. To better reveal the mechanism involved, metabolite analyses were performed. Delta-3 ceftazidime and trans-ceftazidime were regarded as the metabolites of ceftazidime and the metabolite of 7-ACA was regarded as a compound which shared the similar structure with 4-Chlorocinnamic acid. Our study indicated that the green algae performed a satisfactory growth capacity and played a dominant role for the biodegradation of the target antibiotics, which achieved high removal efficiency and low environmental impact.
Determination of hidden hazelnut oil proteins in extra virgin olive oil by cold acetone precipitation followed by in-solution tryptic digestion and MALDI-TOF-MS analysis.[Pubmed:25209075]
J Agric Food Chem. 2014 Oct 1;62(39):9401-9.
Adulteration of extra-virgin olive oil (EVOO) with hazelnut oil (HO) is an illegal practice that could have severe health consequences for consumers due to the possible exposure to hidden hazelnut allergens. Here, matrix-assisted laser-desorption/ionization (MALDI) mass spectrometry (MS) was used as a rapid and sensitive technique for the detection of a low concentration of hazelnut proteins in oil samples. Different protocols were tested for protein extraction, and the most efficient (cold acetone) was applied to HO and EVOO adulterated with HO. The subsequent in-solution tryptic digestion of protein extracts and MALDI-MS analysis, using alpha-cyano-4-Chlorocinnamic acid as matrix, allowed the detection of stable hazelnut peptide markers (i.e., the m/z ions 1002.52, 1356.71, 1394.70, 1440.81, 1453.85, 1555.76, 1629.83, 1363.73, and 1528.67) attributable to the main hazelnut proteins Cor a 9, Cor a 11, and Cor a 1. Thus, the approach might allow the direct detection of specific hazelnut allergens in EVOO at low concentration without time-consuming pretreatments.
MALDI-TOF mass spectrometric determination of intact phospholipids as markers of illegal bovine milk adulteration of high-quality milk.[Pubmed:23232957]
Anal Bioanal Chem. 2013 Feb;405(5):1641-9.
In the dairy industry one of the most common frauds is mixing high-value milk (sheep's and goats') with milk of lower value (cows'). This illegal practice has commercial, ethical, and serious sanitary consequences because consumers can be exposed to hidden allergens contained in the undeclared cows' milk. Here, we investigated the possibility of using matrix-assisted laser-desorption/ionization (MALDI)-time of flight (TOF) mass spectrometry (MS) as a rapid, sensitive, and accurate technique for detection of milk adulteration by analysis of phospholipid profiles. Lipid extracts of pure raw milk, commercial milk, and binary mixtures of cows' and goats' milk and cows' and sheep's milk (the concentrations of each milk varied from 0 % to 50 %) were analyzed with alpha-cyano-4-Chlorocinnamic acid as matrix. The abundance ratio of the ions at m/z 703 and m/z 706 was found to be species-correlated and was used as marker of cows' milk in sheep's and goats' milk. Furthermore, the procedure could potentially be applied to cheese samples, because peaks at m/z 703 and 706 were also found in several commercial cheese samples. This approach proved to be an efficient, rapid, and inexpensive method of detecting milk fraud.
Matching the laser wavelength to the absorption properties of matrices increases the ion yield in UV-MALDI mass spectrometry.[Pubmed:23064675]
Anal Bioanal Chem. 2013 Sep;405(22):6925-32.
A high analytical sensitivity in ultraviolet matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) is only achieved if the laser wavelength corresponds to a high optical absorption of the matrix. Laser fluence and the physicochemical properties of the compounds, e.g., the proton affinity, also influence analytical sensitivity significantly. In combination, these parameters determine the amount of material ejected per laser pulse and the ion yield, i.e., the fraction of ionized biomolecules. Here, we recorded peptide ion signal intensities as a function of these parameters. Three cinnamic acid matrices were investigated: alpha-cyano-4-hydroxycinnamic acid, alpha-cyano-4-Chlorocinnamic acid, and alpha-cyano-2,4-difluorocinnamic acid. In addition, 2,5-dihydroxybenzoic acid was used in comparison experiments. Ion signal intensities "per laser shot" and integrated ion signal intensities were acquired over 900 consecutive laser pulses applied on distinct positions on the dried-droplet sample preparations. With respect to laser wavelength, the two standard MALDI wavelengths of 337/355 nm were investigated. Also, 305 or 320 nm was selected to account for the blue-shifted absorption profiles of the halogenated derivatives. Maximal peptide ion intensities were obtained if the laser wavelength fell within the peak of the absorption profile of the compound and for fluences two to three times the corresponding ion detection threshold. The results indicate ways for improving the analytical sensitivity in MALDI-MS, and in particular for MALDI-MS imaging applications where a limited amount of material is available per irradiated pixel.
Detection of sheep and goat milk adulterations by direct MALDI-TOF MS analysis of milk tryptic digests.[Pubmed:22972782]
J Mass Spectrom. 2012 Sep;47(9):1141-9.
In dairy field, one of the most common frauds is the adulteration of higher value types of milk (sheep's and goat's) with milk of lower value (cow's milk). This illegal practice has an economic advantage for milk producers and poses a threat for consumers' health because of the presence of hidden allergens as, for example, cow milk proteins, in particular, alpha(s1)-casein and beta-lactoglobulin. The urgent need of sensitive techniques to detect this kind of fraud brought to the development of chromatographic, immunoenzymatic, electrophoretic and mass spectrometric assays. In the current work, we present a fast, reproducible and sensitive method based on the direct matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) MS analysis of milk tryptic digests for the detection of milk adulteration by evaluating specie-specific markers in the peptide profiles. Several pure raw and commercial milk samples and binary mixtures containing cows' and goats', cows' and sheep's and goats' and sheep's milk (concentrations of each milk varied from 0% to 100%) were prepared, and tryptic digests were analyzed by MALDI-TOF MS. The use of the new MALDI matrix alpha-cyano-4-Chlorocinnamic acid allowed to detect cow and goat milk peptide markers up to 5% level of adulteration. Finally, from preliminary data, it seems that the strategy could be successfully applied also to detect similar adulterations in cheese samples.
The design and use of a simple System Suitability Test Mix for generic reverse phase high performance liquid chromatography-mass spectrometry systems and the implications for automated system monitoring using global software tracking.[Pubmed:21543072]
J Chromatogr A. 2011 Jun 10;1218(23):3711-7.
The development of a seven-component test mixture designed for use with a generic gradient and a reversed-phase high performance liquid chromatography-mass spectrometry (RP-HPLC-MS) system is discussed. Unlike many test mixtures formulated in order to characterise column quality at neutral pH, the test mixture reported here was designed to permit an overall suitability assessment of the whole liquid chromatography-mass spectrometry (LCMS) system. The mixture is designed to test the chromatographic performance of the column as well as certain aspects of the performance of the individual instrumental components of the system. The System Suitability Test Mix can be used for low and high pH generic reverse phase LCMS analysis. Four phthalates are used: diethyl phthalate (DEP), diamyl phthalate (DAP), di-n-hexyl phthalate (DHP) and dioctyl phthalate (DOP). Three other probes are employed: 8-bromoguanosine (8-BG), amitryptyline (Ami), and 4-Chlorocinnamic acid (4-CCA). We show that analysis of this test mixture can alert the user when any part of the system (instrument or column) contributes to loss of overall performance and may require remedial action and demonstrate that it can provide information that enables us to document data quality control.
MALDI-TOF MS characterization of glycation products of whey proteins in a glucose/galactose model system and lactose-free milk.[Pubmed:21319853]
J Agric Food Chem. 2011 Mar 9;59(5):1793-803.
The major modifications induced by thermal treatment of whey proteins alpha-lactalbumin (alpha-La) and beta-lactoglobulin (beta-Lg) in a model system mimicking lactose-free milk (L(-) sugar mix) were investigated by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS). The analysis of the intact alpha-La revealed species with up to 7 and 14 adducts from lactose and sugar mix, respectively, whereas for beta-Lg 3 and up to 5 sugar moieties were observed in the case of lactose and sugar mix experiments, respectively. A partial enzymatic hydrolysis with endoproteinase AspN prior to mass spectrometric analysis allowed the detection of further modifications and their localization in the amino acid sequence. Using alpha-cyano-4-Chlorocinnamic acid as MALDI matrix, it could be shown that heating alpha-La and beta-Lg with glucose or galactose led to the modification of lysine residues that are not glycated by lactose. The higher glycation degree of whey proteins in a lactose-free milk system relative to normal milk with lactose reflects the higher reactivity of monosaccharides compared to the parent disaccharide. Finally, the analysis of the whey extract of a commercial lactose-free milk sample revealed that the two whey proteins were present as three main forms (native, single, and double hexose adducts).
Synthesis and structure-activity relationships of second-generation hydroxamate botulinum neurotoxin A protease inhibitors.[Pubmed:17951059]
Bioorg Med Chem Lett. 2007 Dec 1;17(23):6463-6.
Botulinum neurotoxins are the most toxic proteins currently known. Based on a recently identified potent lead structure, 2,4-dichlorocinnamic acid hydroxamate, herein we report on the structure-activity relationship of a series of hydroxamate BoNT/A inhibitors. Among them, 2-bromo-4-Chlorocinnamic acid hydroxamate, 2-methyl-4-Chlorocinnamic acid hydroxamate, and 2-trifluoromethyl-4-Chlorocinnamic acid hydroxamate displayed comparable inhibitory activity to that of the lead structure.
A novel ortho-dehalogenation reaction of 2-chlorocinnamic acid catalyzed by the pink yeast Rhodotorula rubra Y-1529.[Pubmed:10789983]
Chemosphere. 2000 Jun;40(12):1417-25.
In the present study, a resting cells suspension of Rhodotorula rubra Y-1529 was shown to have the capacity to perform an ortho-dehalogenation reaction on 2-chlorocinnamic acid. The results from the biodegradation of U-[14C]benzoic acid, cinnamic acid, 3-chlorocinnamic acid and 4-Chlorocinnamic acid suggest that the first step of the ortho-dehalogenation reaction occurred during the oxidation of the unsaturated C3 side chain of 2-chlorocinnamic acid to 2-chlorobenzoic acid. None of the 2-chlorobenzoic acid was found in the biodegradation system, suggesting that this step was a highly regulated step. After the side-chain oxidation reaction, the hydroxylation of the benzene ring was determined to be at the para-position first, followed by the meta-position. The occurrence of 3:4-position ring fission reactions and the production of the final product, CO2, was proven by the biodegradation of U-[14C] benzoic acid. This oxidative dehalogenation reaction catalyzed by R. rubra was found to be regiospecific for 2-chlorocinnamic acid; the chloride ion was probably removed after the ring fission reaction. A pathway of the ortho-dehalogenation reaction of 2-chlorocinnamic acid catalyzed by R. rubra was proposed based on these data.
Enzymatic dehalogenation of 4-chlorobenzoate by extracts from Arthrobacter sp. SU DSM 20407.[Pubmed:3223988]
Biol Chem Hoppe Seyler. 1988 Jul;369(7):567-71.
In extracts from Arthrobacter sp. SU DSM 20407 an enzyme was detectable, that converted 4-chlorobenzoate into 4-hydroxybenzoate. This conversion was also observed when no oxygen was present in the reaction mixture. Boiling for 5 min destroyed the enzyme activity. 4-Bromo- and 4-iodobenzoate were substrates for the enzyme too, but not 4-fluorobenzoate, 4-chlorophenylacetate and 4-Chlorocinnamic acid. The enzyme showed optimum activity at 16 degrees C and at pH 7-7.5. The specific activity in the extracts varied between 0.5 and 5 mU/mg of protein. Zn2+ and Cu2+ inhibited the enzyme, while H2O2 slightly activated. In contrast to all other 4-chlorobenzoate dehalogenases described before the enzyme was not inhibited by EDTA, nor was it activated by Mn2+. Other divalent ions also had no effect. The molecular mass of the enzyme was 45,000 +/- 5,000 Da as judged by gel-filtration.
Chloroperoxidase-catalyzed halogenation of trans-cinnamic acid and its derivatives.[Pubmed:4044583]
J Biol Chem. 1985 Oct 5;260(22):11962-9.
Several 2,3-unsaturated carboxylic acids, such as trans-cinnamic acid and its derivatives, were found to be halogenated by chloroperoxidase of Caldariomyces fumago in the presence of hydrogen peroxide and either Cl- or Br-. Cinnamic acid, 4-hydroxycinnamic acid, 4-methoxycinnamic acid, and 3,4-dimethoxycinnamic acid were suitable substrates of chloroperoxidase, and were converted to 2-halo-3-hydroxycarboxylic acid, 2,3-dihydroxycarboxylic acid, decarboxylated halohydrin, or decarboxylated halocompound. However, 4-nitrocinnamic acid and 4-Chlorocinnamic acid having electron-attracting groups did not serve as a substrate of the enzyme. The enzyme also did not act on acrylic acid, acrylamide, crotonic acid, fumaric acid, etc. From these data, the enzymatic reactions of chloroperoxidase, concerning the substrate specificity, stereoselectivity, and the reaction mechanism, are discussed on the basis of current knowledge regarding the reaction mechanism of the enzyme. Also they are compared with the chemical reactions of molecular halogen and hypohalous acid.


