SIN-1 chlorideWater-soluble NO donor CAS# 16142-27-1 |
- Atorvastatin Calcium
Catalog No.:BCC2319
CAS No.:134523-03-8
- Pitavastatin
Catalog No.:BCC4140
CAS No.:147511-69-1
- Pitavastatin Calcium
Catalog No.:BCC3842
CAS No.:147526-32-7
- Clinofibrate
Catalog No.:BCC5020
CAS No.:30299-08-2
- Mevastatin
Catalog No.:BCN2568
CAS No.:73573-88-3
- Lovastatin
Catalog No.:BCN1060
CAS No.:75330-75-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 16142-27-1 | SDF | Download SDF |
| PubChem ID | 197942 | Appearance | Powder |
| Formula | C6H11ClN4O2 | M.Wt | 206.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 3-Morpholinylsydnoneimine chloride | ||
| Solubility | Soluble to 100 mM in water and to 100 mM in DMSO | ||
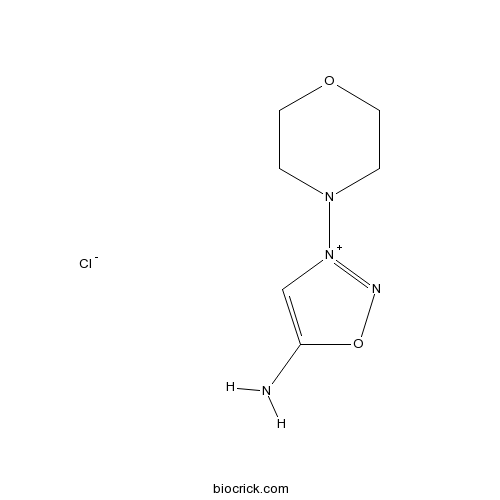
| Chemical Name | 3-morpholin-4-yloxadiazol-3-ium-5-amine;chloride | ||
| SMILES | C1COCCN1[N+]2=NOC(=C2)N.[Cl-] | ||
| Standard InChIKey | ZRFWHHCXSSACAW-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C6H11N4O2.ClH/c7-6-5-10(8-12-6)9-1-3-11-4-2-9;/h5H,1-4,7H2;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | This compound (the active product of the prodrug SIN-10, molsidomine) acts as a vasodilator and inhibitor of platelet aggregation; longer acting than nitroprusside or nitroglycerin. Decreases myocardial necrosis and reperfusion-induced endothelial dysfunction in models of myocardial ischemia-reperfusion. |

SIN-1 chloride Dilution Calculator

SIN-1 chloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.8396 mL | 24.1978 mL | 48.3957 mL | 96.7914 mL | 120.9892 mL |
| 5 mM | 0.9679 mL | 4.8396 mL | 9.6791 mL | 19.3583 mL | 24.1978 mL |
| 10 mM | 0.484 mL | 2.4198 mL | 4.8396 mL | 9.6791 mL | 12.0989 mL |
| 50 mM | 0.0968 mL | 0.484 mL | 0.9679 mL | 1.9358 mL | 2.4198 mL |
| 100 mM | 0.0484 mL | 0.242 mL | 0.484 mL | 0.9679 mL | 1.2099 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ABT
Catalog No.:BCC7998
CAS No.:1614-12-6
- HTH-01-015
Catalog No.:BCC4010
CAS No.:1613724-42-7
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Fmoc-Chg-OH
Catalog No.:BCC3164
CAS No.:161321-36-4
- 1-O-Acetyl-6-O-isobutyrylbritannilactone
Catalog No.:BCN7795
CAS No.:1613152-34-3
- SR-9243
Catalog No.:BCC3983
CAS No.:1613028-81-1
- H-Orn(Z)-OtBu.HCl
Catalog No.:BCC2677
CAS No.:161234-80-6
- Cimicidanol 3-O-alpha-L-arabinoside
Catalog No.:BCN6528
CAS No.:161207-05-2
- H-Asp(OEt)-OEt.HCl
Catalog No.:BCC2888
CAS No.:16115-68-7
- Bidwillol A
Catalog No.:BCN4858
CAS No.:161099-42-9
- Cimicifugoside H2
Catalog No.:BCN7949
CAS No.:161097-77-4
- Formyl-DL-Trp-OH
Catalog No.:BCC3120
CAS No.:16108-03-5
- 7-NINA
Catalog No.:BCC5674
CAS No.:161467-34-1
- 4-Chlorocinnamic acid
Catalog No.:BCN5032
CAS No.:1615-02-7
- Simiarenol
Catalog No.:BCN1714
CAS No.:1615-94-7
- Fmoc-Alaninol
Catalog No.:BCC2729
CAS No.:161529-13-1
- Cyclo (-RGDfK)
Catalog No.:BCC3590
CAS No.:161552-03-0
- Ro 48-8071
Catalog No.:BCC5545
CAS No.:161582-11-2
- (S)-N-Glycidylphthalimide
Catalog No.:BCN3815
CAS No.:161596-47-0
- 2'',3''-Di-O-acetyl-5''-deoxy-5-fuluro-D-cytidine
Catalog No.:BCN1545
CAS No.:161599-46-8
- beta-Amyrin acetate
Catalog No.:BCN1715
CAS No.:1616-93-9
- ZK 200775
Catalog No.:BCC7339
CAS No.:161605-73-8
- Epimedonin B
Catalog No.:BCN7889
CAS No.:1616061-69-8
- Hispidanin B
Catalog No.:BCN7394
CAS No.:1616080-84-2
Novel SIN-1 reactive intermediates modulate chloride secretion across murine airway cells.[Pubmed:12957658]
Free Radic Biol Med. 2003 Sep 15;35(6):662-75.
We examined the effects of reactive oxygen-nitrogen intermediates on chloride (Cl-) currents across murine tracheal epithelial (MTE) cells isolated from CD-1 mice. MTE cells were cultured on permeable supports until they formed water-tight monolayers with transepithelial resistances (Rt)>500 Omega/cm2 and then were mounted in Ussing chambers. Baseline short-circuit current (ISC) values, prior to and following the addition of 10 microM amiloride (an inhibitor of sodium-transport pathways) into the apical side, were 65 +/- 1.9 microA/cm2 and 7.6 +/- 0.51 microA/cm2, respectively (X +/- 1 SE, n=32). The addition of 3-morpholinosydnominine (SIN-1, 1 mM), which generates both superoxide and nitric oxide anions, to amiloride-treated monolayers resulted in a transient increase of ISC to a peak value of 35 +/- 1.3 microA/cm2 (X +/- SE, n=14) within the next 30-60 min. After this, the ISC decreased gradually and returned to its pre-SIN-1 value. These changes were blocked by glibenclamide (200 microM), an inhibitor of cystic fibrosis transmembrane regulator, or reduced by glutathione (GSH, 5 mM), a scavenger of peroxynitrite. Forskolin (10 microM) augmented the SIN-1 effect when added at the peak of the SIN-1 response but not when ISC had returned to its baseline value. Perfusion of MTE cells with SIN-1 also increased whole cell Cl- currents 4-fold and the open probability of CFTR-type single-channel currents from 0.041 to 0.92 in a transient fashion. Decomposed SIN-1, but not pure SIN-1c (the stable decomposition product of SIN-1), also increased ISC with an EC50 of 5 microM. Electrospray mass spectroscopy revealed several unique and uncharacterized compounds formed during the decomposition of SIN-1 as well as the reaction of SIN-1c with peroxynitrite. Formation of these compounds was inhibited by GSH. We conclude that compounds formed by the reaction of peroxynitrite with by-products of SIN-1, rather than reactive oxygen-nitrogen species per se, were responsible for the modulation of Cl- secretion across primary cultures of MTE cells.
Cardioprotection and attenuation of endothelial dysfunction by organic nitric oxide donors in myocardial ischemia-reperfusion.[Pubmed:1738117]
J Pharmacol Exp Ther. 1992 Feb;260(2):668-75.
The effects of two nitric oxide (NO) donors were evaluated in a 6-h model of feline myocardial ischemia-reperfusion. After 80 min of a 90-min ischemic period, SIN-1 or C87-3754 or their respective controls (i.e., 0.9% NaCl or C88-3934, a control compound which does not release NO) were given i.v. as a bolus (0.1 mg/kg) and infused at 1 mg/kg/h for the entire 4.5-h reperfusion period. Administration of the active NO donors significantly decreased the necrotic area/area-at-risk ratio from 29 +/- 3% in the vehicle group to 9 +/- 2 and 11 +/- 5% in the SIN-1 and C87-3754 groups, respectively (P less than .001). The inactive NO donor C88-3934 failed to reduce infarct size (31 +/- 3%). Neither NO donor reduced the accumulation of neutrophils in the necrotic area when compared to their respective control groups, but both agents significantly attenuated coronary endothelial dysfunction as shown by a vasorelaxation to acetylcholine of 62 +/- 2 and 64 +/- 3% in the SIN-1- and C87-3754-treated arteries, as compared to only a 27 +/- 3 and 34 +/- 4% vasorelaxation in the vehicle and inactive NO donor groups, respectively (P less than .001). Our studies show that SIN-1 and C87-3754 exert beneficial effects in a 6-h model of myocardial ischemia-reperfusion. Both NO donors decreased myocardial necrosis and decreased the reperfusion-induced endothelial dysfunction without significantly altering the pressure-rate index (i.e., an index of myocardial oxygen demand).
Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP.[Pubmed:2160060]
Mol Pharmacol. 1990 May;37(5):671-81.
We investigated the roles of cyclic GMP and cyclic AMP in the inhibition of rabbit platelet aggregation and degranulation by two nitrovasodilators, sodium nitroprusside (SNP) and 3-morpholinosydnonimine (SIN-1; the active metabolite of molsidomine), with particular reference to the synergistic interaction of these drugs with prostaglandin E1 (PGE1). Changes in platelet cyclic [3H]GMP and cyclic [3H]AMP were measured by rapid and sensitive prelabeling techniques, the validity of which were confirmed by radioimmunoassays. Incubation of the platelets with 0.1 to 10 microM SNP alone for 0.5 min caused progressively greater inhibitions of platelet function associated with large dose-dependent increases in cyclic [3H]GMP and 1.4- to 3.0-fold increases in cyclic [3H]AMP. However, addition of SNP with the adenylate cyclase activator, PGE1, at a concentration of the latter that had little effect alone, caused much larger increases in cyclic [3H]AMP and greatly enhanced the inhibition of platelet aggregation. SIN-1 had effects similar to those of SNP, although it was less active. The adenylate cyclase inhibitor 2',5'-dideoxyadenosine (DDA) diminished the increases in cyclic [3H]AMP caused by SNP or SIN-1 in both the presence and absence of PGE1 but reduced the inhibition of platelet function caused by the nitrovasodilators only in the presence of PGE1. These results suggest that, although cyclic GMP may mediate the inhibition of rabbit platelet function by high concentrations of nitrovasodilators added alone, the synergistic interaction of lower concentrations with PGE1 depends on an enhanced accumulation of cyclic AMP. Synergistic effects on cyclic [3H]AMP accumulation were also observed on incubation of platelets with SNP and adenosine, another activator of adenylate cyclase. Hemoglobin, which binds nitric oxide, blocked or reversed the increases in both cyclic [3H]GMP and cyclic [3H]AMP in platelets caused by the nitrovasodilators added either alone or with PGE1. Cilostamide, a selective inhibitor of platelet low Km cyclic AMP phosphodiesterase, had effects on platelet cyclic [3H]AMP accumulation identical to those of SNP, suggesting that the action of the latter depends on inhibition of the same enzyme. M&B 22,948, a selective inhibitor of cyclic GMP phosphodiesterase, potentiated the increases in both cyclic [3H]GMP and cyclic [3H]AMP caused by SNP. A hyperbolic relationship was found between the increases in cyclic [3H]GMP and cyclic [3H]AMP caused by different concentrations of SNP; this relationship was not affected by addition of M&B 22,948. The results strongly suggest that the increases in platelet cyclic [3H]AMP caused by nitrovasodilators in the presence or absence of activators of adenylate cyclase are mediated by the inhibition by cyclic GMP of cyclic AMP breakdown.(ABSTRACT TRUNCATED AT 400 WORDS)


